Page 308 - Gas Purification 5E
P. 308
292 Gas Purification
TEMPERATURE 'C
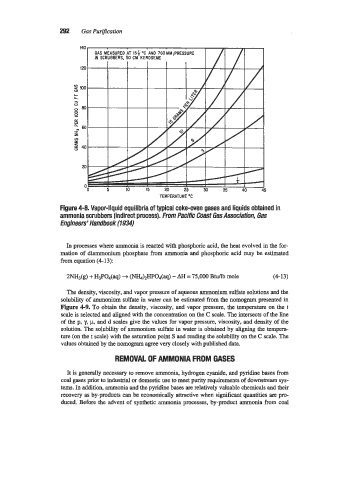
Figure 4-8. Vapor-liquid equilibria of typical coke-oven gases and liquids obtained in
ammonia scrubbers (indirect process). From Pacific Coast Gas Assmiafion, Gas
Engineers' Handbook (1934)
In processes where ammonia is reacted with phosphoric acid, the heat evolved in the for-
mation of diammonium phosphate from ammonia and phosphoric acid may be estimated
from equation (4- 13):
2NH3(g) + H3P04(aq) + (NH&HP04(aq) - AH = 75,000 Btu/lb mole (4- 13)
The density, viscosity, and vapor pressure of aqueous ammonium sulfate solutions and the
solubility of ammonium sulfate in water can be estimated from the nomogram presented in
Figure 4-9. To obtain the density, viscosity, and vapor pressure, the temperature on the t
scale is selected and aligned with the concentration on the C scale. The intersects of the line
of the p, y, p, and d scales give the values for vapor pressure, viscosity, and density of the
solution. The solubility of ammonium sulfate in water is obtained by aligning the tempera-
ture (on the t scale) with the saturation point S and reading the solubility on the C scale. The
values obtained by the nomogram agree very closely with published data.
REMOVAL OF AMMONIA FROM GASES
It is generally necessary to remove ammonia, hydrogen cyanide, and pyridine bases from
coal gases prior to industrial or domestic use to meet purity requirements of downstream sys-
tems. In addition, ammonia and the pyridine bases are relatively valuable chemicals and their
recovery as by-products can be economically attractive when significant quantities are pro-
duced. Before the advent of synthetic ammonia processes, by-product ammonia from coal