Page 74 - Gas Purification 5E
P. 74
64 Gus Pur@cation
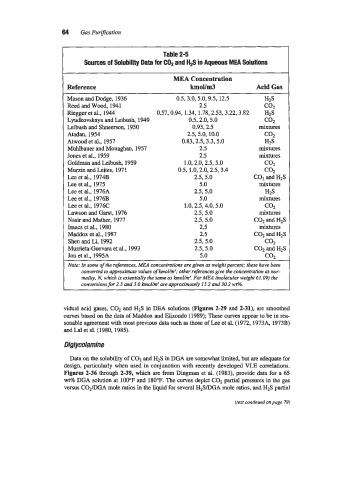
Table 2-5
Sources of Solubility Data for COP and H2S in Aqueous MEA Solutions
lMEA Concentration
Reference kmoYrn3 Acid Gas
Mason and Dodge, 1936 0.5,3.0,5.0,9.5, 12.5 h25
Reed and Wood, 1941 2.5 co.,
Riegger et al., 1944 0.57,0.94, 1.34, 1.78,2.53,3.22,3.82 H2S
Lyudkovskaya and Leibush, 1949 0.5,2.0,5.0 c02
Leibush and Shneerson, 1950 0.93, 2.5 mixtures
Atadan, 1954 2.5,5.0, 10.0 c02
Atwood et al., 1957 0.83,2.5,3.3,5.0 HZS
Muhlbauer and Monaghan, 1957 2.5 mixtures
Jones et al., 1959 2.5 mixtures
Goldman and Leibush, 1959 1.0,2.0,2.5,5.0 coz
Murzin and kites, 197 1 0.5, 1.0,2.0,2.5,3.4 c02
Lee et al., 1974B 2.5,5.0 COz and H2S
Lee et al., 1975 5.0 mixtures
Lee et al., 1976A 2.5, 5.0 h25
Lee et al., 1976B 5 .O mixtlUeS
Lee et al., 1976C 1.0,2.5,4.0,5.0 c02
Lawson and Garst, 1976 2.5,5.0 mixtlUeS
Nasir and Mather, 1977 2.5, 5.0 C02 and HIS
Isaacs et al., 1980 2.5 mixtures
Maddox et al., 1987 2.5 COz and H2S
Shen and Li, 1992 255.0 c02
Murrieta-Guevara et al., 1993 2.5,5.0 C02 and HlS
Jou et al., 1995A 5.0 coz
Note: In some of the references, MEA concentrations are given as weight percent: these haw been
converted to approximate values of hol/m’; other references give the concentration as nor-
mality, N. which is essentially the same as h01/m3. For MEA (molecular weight 61.09) the
conversions for 2.5 and 5.0 hoU& are approximately 15.2 and 30.2 wPA.
vidual acid gases, COz and H2S in DEA solutions (Figures 2-29 and 2-31), are smoothed
curves based on the data of Maddox and Eliondo (1989); These curves appear to be in rea-
sonable agreement with most previous data such as those of Lee et al. (l972,1973A, 1973B)
and Lal et al. (1980, 1985).
Diglycolamine
Data on the solubility of COz and H2S in DGA are somewhat limited, but are adequate for
design, particularly when used in conjunction with recently developed VLE correlations.
Figures 2-36 through 2-39, which are from Dingman et al. (1983), provide data for a 65
wt% DGA solution at 100°F and 180°F. The curves depict C02 partial pressures in the gas
versus C02/DGA mole ratios in the liquid for several H2S/DGA mole ratios, and H2S partial
(text continued on page 79)