Page 82 - Gas Purification 5E
P. 82
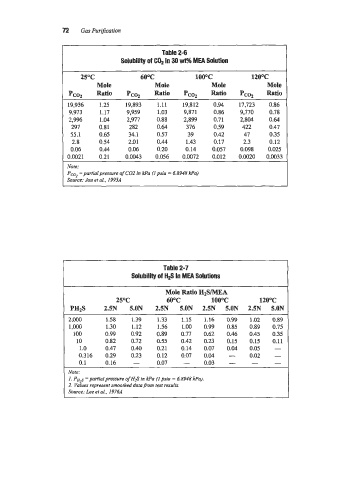
72 Gas Purification
~ ~~ ~
Table 2-6
Solubility of COP in 30 wt% MEA Solution
~~ ~ ~ ~
25OC 60°C lOo0C 120%
Mole Mole Mole Mole
Pcoz Ratio Pcoz Ratio Pcs Ratio Pcoz Ratio
19,936 1.25 19,893 1.11 19,812 0.94 17,723 0.86
9,973 1.17 9,959 1.03 9,871 0.86 9,770 0.78
2,996 1.04 2,977 0.88 2,899 0.71 2,804 0.64
297 0.81 282 0.64 376 0.59 422 0.47
55.1 0.65 34.1 0.57 39 0.42 47 0.35
2.8 0.54 2.01 0.44 1.43 0.17 2.3 0.12
0.06 0.44 0.06 0.20 0.14 0.057 0.098 0.025
0.0021 0.21 0.0043 0.056 0.0072 0.012 0.0020 0.0033
:Vote:
Pm2 =partial pressure of C02 in kPa (I psia = 6.8948 Pa)
Source: Jou et al., 199jA
Table 2-7
Solubility of H# in MEA Solutlons
Mole Ratio HZSMEA
25OC 6OoC lO0OC l2OOC
PH2S 2.5N 5.ON 2.5N 5.ON 2.5N 5.ON 2.5N 5.0N
2,000 1.58 1.39 1.33 1.15 1.16 0.99 1.02 0.89
1,OoO 1.30 1.12 1.56 1.00 0.99 0.85 0.89 0.75
100 0.99 0.92 0.89 0.77 0.62 0.46 0.45 0.35
10 0.82 0.72 0.55 0.42 0.23 0.15 0.15 0.11
1 .o 0.47 0.40 0.21 0.14 0.07 0.04 0.05 -
0.316 0.29 0.23 0.12 0.07 0.04 - 0.02 -
0.1 0.16 - 0.07 - 0.03 - - -
-
~ ~ - ~~~~~
Note:
I. PH~ =partial presswe of €€$ m kPa (1 psi0 = 6.8948 Pa). 1
2. Values represent smoothed data-fmm test mI&.
Source: Lee et al., I976A