Page 83 - Gas Purification 5E
P. 83
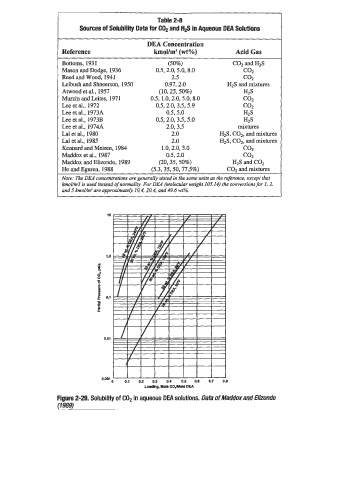
Table 2-8
Sources of Solubility Data for C02 and H# in Aqueous DEA Solutions
DEA Concentration
Reference km0Ym3 (wt”/) Acid Gas
Bottoms, 1931 (50%) COZ and H2S
Mason and Dodge, 1936 0.5, 2.0, 5.0, 8.0 COZ
Reed and Wood, 1941 2.5 COZ
Leibush and Shneerson, 1950 0.97,2.0 HzS and mixtures
Atwood et al., 1957 (10,25,508) h25
Murzin and Leites, 1971 0.5, 1.0,2.0,5.0,8.0 c02
Lee et al., 1972 0.5,2.0,3.5,5.9 COZ
Lee et al., 1973A 0.5,5.0 HZS
Lee et al., 1973B 0.5,2.0,3.5,5.0 HIS
Lee et al., 1974A 2.0, 3.5 mixtures
La1 et al., 1980 2.0 HzS, COz, and mixtures
Lal et al., 1985 2.0 HzS, COz, and mixtures
Kennard and Meisen, 1984 1 .O, 2.0, 3.0 COZ
Maddox et al., 1987 0.5,2.0 c02
Maddox and Elizondo, 1989 (20,35,50%) H2S and COP
Ho and Eguren, 1988 (5.3,35,50,77.5%) COZ and mixtures
Note: The DEA concentrations are generally stated in the same units as the reference, except that
kmoUm3 is used instead of normali$ For DEA (molecular weight 105.14) the conversions for I, 2.
and 5 kmol/mJ are approximately 10.4,20.4, and 49.4 wt%.
Figure 2-29. Solubility of COP in aqueous DEA solutions. Data of Maddoxand Elizondo
(19as)