Page 79 - Gas Adsorption Equilibria
P. 79
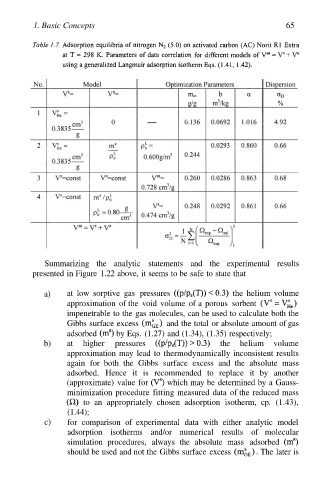
1. Basic Concepts 65
Summarizing the analytic statements and the experimental results
presented in Figure 1.22 above, it seems to be safe to state that
a) at low sorptive gas pressures the helium volume
approximation of the void volume of a porous sorbent
impenetrable to the gas molecules, can be used to calculate both the
Gibbs surface excess and the total or absolute amount of gas
adsorbed by Eqs. (1.27) and (1.34), (1.35) respectively;
b) at higher pressures the helium volume
approximation may lead to thermodynamically inconsistent results
again for both the Gibbs surface excess and the absolute mass
adsorbed. Hence it is recommended to replace it by another
(approximate) value for which may be determined by a Gauss-
minimization procedure fitting measured data of the reduced mass
to an appropriately chosen adsorption isotherm, cp. (1.43),
(1.44);
c) for comparison of experimental data with either analytic model
adsorption isotherms and/or numerical results of molecular
simulation procedures, always the absolute mass adsorbed
should be used and not the Gibbs surface excess The later is