Page 110 - Geochemical Remote Sensing of The Sub-Surface
P. 110
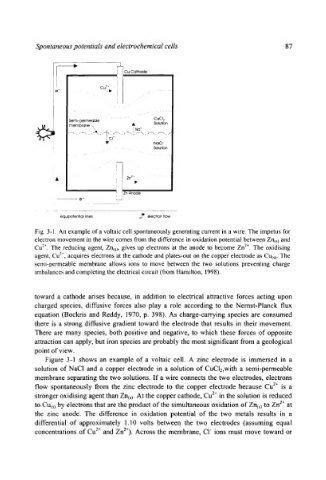
Spontaneous potentials and electrochemical cells 87
r
Cu Cathode
.. .. Cu 2+ ..........
. ...... 9
. . . . . . . . .
... .......
.... 9 -.. . ..................
............. CuCI~
Semi-permeable
membrane ,\ ~Na. Solution
CI"
NoCI
. . . . .. Solution
...
.,_ .. .....
[ ',
i ,.
F-- 1 Zn'2 "i
! | Zn Anode
e- 1
equipotenttal lines e-r~ electron flow
Fig. 3-1. An example of a voltaic cell spontaneously generating current in a wire. The impetus for
electron movement in the wire comes from the difference in oxidation potential between Zn(s) and
Cu 2+. The reducing agent, Zn(s), gives up electrons at the anode to become Zn 2+. The oxidising
agent, Cu 2~, acquires electrons at the cathode and plates-out on the copper electrode as Cu(~). The
semi-permeable membrane allows ions to move between the two solutions preventing charge
imbalances and completing the electrical circuit (from Hamilton, 1998).
toward a cathode arises because, in addition to electrical attractive forces acting upon
charged species, diffusive forces also play a role according to the Nemst-Planck flux
equation (Bockris and Reddy, 1970, p. 398). As charge-carrying species are consumed
there is a strong diffusive gradient toward the electrode that results in their movement.
There are many species, both positive and negative, to which these forces of opposite
attraction can apply, but iron species are probably the most significant from a geological
point of view.
Figure 3-1 shows an example of a voltaic cell. A zinc electrode is immersed in a
solution of NaCI and a copper electrode in a solution of CuCl2,with a semi-permeable
membrane separating the two solutions. If a wire connects the two electrodes, electrons
flow spontaneously from the zinc electrode to the copper electrode because Cu 2+ is a
stronger oxidising agent than Zn(~). At the copper cathode, Cu 2+ in the solution is reduced
to Cu(~) by electrons that are the product of the simultaneous oxidation of Zn(s) to Zn 2+ at
the zinc anode. The difference in oxidation potential of the two metals results in a
differential of approximately 1.10 volts between the two electrodes (assuming equal
concentrations of Cu 2+ and Zn2+). Across the membrane, C1 ~ ions must move toward or