Page 113 - Geochemical Remote Sensing of The Sub-Surface
P. 113
90 S.M. Hamilton
this principle in the case of natural reactions that involve either H + or OH. In nature, a
molar concentration of either H + or OH- is exceptionally high relative to a molar
concentration of many of the other species that they are likely to react with. As such, the
standard conditions used in the determination of standard electrode potentials result in an
exaggeration of the potential concentrations of H + and OH relative to many other
naturally-occurring reactants.
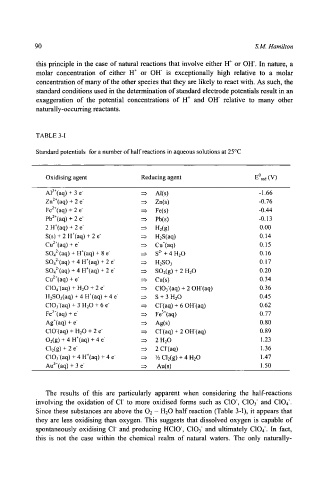
TABLE 3-I
Standard potentials for a number of half reactions in aqueous solutions at 25~
Oxidising agent Reducing agent EOred (V)
Al3+(aq) + 3 e ~ Al(s) -1.66
Zn2+(aq) + 2 e :::> Zn(s) -0.76
Fe2+(aq) + 2 e :::> Fe(s) -0.44
pb2+(aq) + 2 e :z> Pb(s) -0.13
2 H+(aq) + 2 e ::> Hz(g) 0.00
S(s) + 2 H+(aq) + 2 e- ::> H2S(aq) 0.14
Cu2+(aq) + e :::> Cu+(aq) 0.15
SO42(aq) + H+(aq) + 8 e- :z> S 2- + 4 H20 0.16
SO42(aq) + 4 H+(aq) + 2 e ~ H2SO3 0.17
SO42(aq) + 4 H+(aq) + 2 e ~ SO2(g) + 2 H20 0.20
Cu2+(aq) + e ::z, Cu(s) 0.34
CIO4(aq) + H20 + 2 e =:, CIO3(aq) + 20H(aq) 0.36
H2SO3(aq) + 4 H+(aq) + 4 e- ~ S + 3 H20 0.45
CIO3(aq) + 3 H20 + 6 e ::::, Cl(aq) + 60H(aq) 0.62
Fe3~(aq) + e- ::::, Fe3+(aq) 0.77
Ag+(aq) + e ::, Ag(s) 0.80
CIO-(aq) + H20 + 2 e :::::, Cl(aq) + 20H(aq) 0.89
O2(g) + 4 H+(aq) + 4 e ~ 2 H20 1.23
Cl2(g) + 2 e- ::> 2 Cl(aq) 1.36
CIO3-(aq) + 4 H+(aq) + 4 e- :::, 89 Cl2(g) + 4 H20 1.47
Au3+(aq) + 3 e :::, Au(s) 1.50
The results of this are particularly apparent when considering the half-reactions
involving the oxidation of CI to more oxidised forms such as CIO, C103 and C104.
Since these substances are above the 02 - H20 half reaction (Table 3-I), it appears that
they are less oxidising than oxygen. This suggests that dissolved oxygen is capable of
spontaneously oxidising CI and producing HCIO, CIO3 and ultimately C104. In fact,
this is not the case within the chemical realm of natural waters. The only naturally-