Page 116 - Geochemical Remote Sensing of The Sub-Surface
P. 116
Spontaneous potentials and electrochemical cells 93
Since not all redox-active solutions contain such reversible redox couples (Drever,
1982), ORP probes may not always provide reproducible or accurate results (Bartlett and
James, 1995). A related problem occurs due to the build-up of deposits on the platinum
during these reactions, leading to memory effects in the probe when testing other
solutions. These cause long-term drift in the readings, particularly when testing a sample
of low ionic strength after one with high ionic strength. Furthermore, many natural redox
couples have slow rates of reaction, which result in slow response and instrument drift
during ORP measurement. Consequently, ORP probes are often used to obtain a
qualitative idea of the redox composition of waters or sediments but are seldom used to
obtain quantitative SP measurements between two areas.
By far the most common way to measure SP is to use a millivolt meter connected
between two non-polarising electrodes placed at different points on the ground. A
measured voltage difference would usually equal the spontaneous potential between the
two points. Non-polarising electrodes are important because they must be equally
capable of receiving and discharging electrons. The type that is almost invariably used
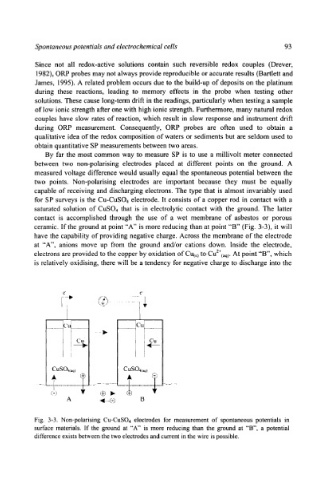
for SP surveys is the Cu-CuSO4 electrode. It consists of a copper rod in contact with a
saturated solution of CuSO4 that is in electrolytic contact with the ground. The latter
contact is accomplished through the use of a wet membrane of asbestos or porous
ceramic. If the ground at point "A" is more reducing than at point "B" (Fig. 3-3), it will
have the capability of providing negative charge. Across the membrane of the electrode
at "A", anions move up from the ground and/or cations down. Inside the electrode,
electrons are provided to the copper by oxidation of Cuts ) to Cu2+(aq). At point "B", which
is relatively oxidising, there will be a tendency for negative charge to discharge into the
e-
k
l
c i cu
CuSO4~,~ ~)1
CuSO4~q~
........ , .............................
(-) (.4-) I~
A ,ql--(-) B
Fig. 3-3. Non-polarising Cu-CuSO4 electrodes for measurement of spontaneous potentials in
surface materials. If the ground at "A" is more reducing than the ground at "B", a potential
difference exists between the two electrodes and current in the wire is possible.