Page 119 - Geochemical Remote Sensing of The Sub-Surface
P. 119
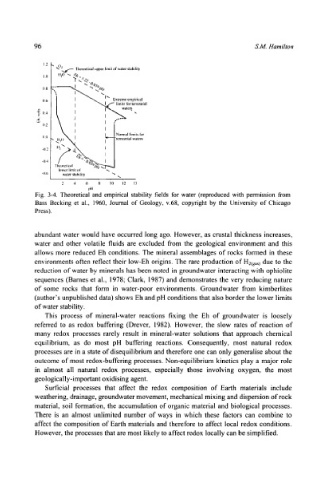
96 S.M. Hamilton
1.2 x ~O~.__ Theoretical upper limit of water stability
!.(i JiO -- r
"r ~/..,.,
9 I ..0%
0.8 :;," ~ x
I~ Extreme empirical
0.6 I ~ "x,,,.Ld"" limits tbr terrestrial
I I ~ ters ..
I
0.4 as,..--
(1.2 i
,,0 , '
-0.2 II2N "~
-0.4 ., , / , " O. ~ ~
Jncorctical -,ojt ,~
lower limit of
-0.6 water stability
2 4 6 8 i0 12 13
pl!
Fig. 3-4. Theoretical and empirical stability fields for water (reproduced with permission from
Bass Becking et al., 1960, Journal of Geology, v.68, copyright by the University of Chicago
Press).
abundant water would have occurred long ago. However, as crustal thickness increases,
water and other volatile fluids are excluded from the geological environment and this
allows more reduced Eh conditions. The mineral assemblages of rocks formed in these
environments often reflect their low-Eh origins. The rare production of H2(g,s) due to the
reduction of water by minerals has been noted in groundwater interacting with ophiolite
sequences (Barnes et al., 1978; Clark, 1987) and demonstrates the very reducing nature
of some rocks that form in water-poor environments. Groundwater from kimberlites
(author's unpublished data) shows Eh and pH conditions that also border the lower limits
of water stability.
This process of mineral-water reactions fixing the Eh of groundwater is loosely
referred to as redox buffering (Drever, 1982). However, the slow rates of reaction of
many redox processes rarely result in mineral-water solutions that approach chemical
equilibrium, as do most pH buffering reactions. Consequently, most natural redox
processes are in a state of disequilibrium and therefore one can only generalise about the
outcome of most redox-buffering processes. Non-equilibrium kinetics play a major role
in almost all natural redox processes, especially those involving oxygen, the most
geologically-important oxidising agent.
Surficial processes that affect the redox composition of Earth materials include
weathering, drainage, groundwater movement, mechanical mixing and dispersion of rock
material, soil formation, the accumulation of organic material and biological processes.
There is an almost unlimited number of ways in which these factors can combine to
affect the composition of Earth materials and therefore to affect local redox conditions.
However, the processes that are most likely to affect redox locally can be simplified.