Page 123 - Geochemical Remote Sensing of The Sub-Surface
P. 123
1 O0 S.M. Hamilton
lz;arth'S' surtace "."
(mV)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-600
. +400
iii iii ; iiiiiiiiiiii
u,I
O
!iiii )ill iiiiiiill iiiii.
i '
I
7'
-400
Earth's Lower Crust
_ _ . .
Increasing Eh
H < d (t~ < c 0~ < b 9 < a (-~<O Positive Charge Carriers
H < D <-'~ <C (-3 <B (-) <A ~) <O Negative Charge Carriers
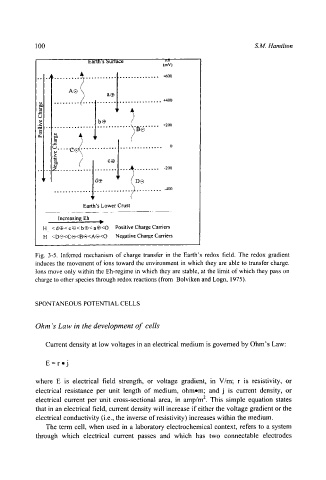
Fig. 3-5. Inferred mechanism of charge transfer in the Earth's redox field. The redox gradient
induces the movement of ions toward the environment in which they are able to transfer charge.
Ions move only within the Eh-regime in which they are stable, at the limit of which they pass on
charge to other species through redox reactions (from Bolviken and Logn, 1975).
SPONTANEOUS POTENTIAL CELLS
Ohm's Law in the development of cells
Current density at low voltages in an electrical medium is governed by Ohm's Law:
E=r,j
where E is electrical field strength, or voltage gradient, in V/m; r is resistivity, or
electrical resistance per unit length of medium, ohmem; and j is current density, or
electrical current per unit cross-sectional area, in amp/m 2. This simple equation states
that in an electrical field, current density will increase if either the voltage gradient or the
electrical conductivity (i.e., the inverse of resistivity) increases within the medium.
The term cell, when used in a laboratory electrochemical context, refers to a system
through which electrical current passes and which has two connectable electrodes