Page 127 - Geochemical Remote Sensing of The Sub-Surface
P. 127
104 S.M. Hamilton
" 'k i
[ .t t.- ~ ..... J
... \\ \ "
I
+200 T " -." 7(~)--) "
/ ~ ..- 4 ..... I. ' ........ "~ E.I
+100 -- ~ " . . . . . . . . . . . . . . . . .'3"-
§
.. J ..~. . . . . . . . O
, (e)
mV - i . . . . . . . . . . ~ .
/ ,--
l
J
......... _~. ~...
/
1 O0 - / .~, (5)
" :/ ~ // .. .- ,
/ i ; . . .. .
-200
, . j . . . . .
..-L0~.. Equipotentiai lines ~, Electron flow
.,- Negative Current flow Sulfide
Positive/negative ion movement Im
~,q: Cathode r.X) Anode
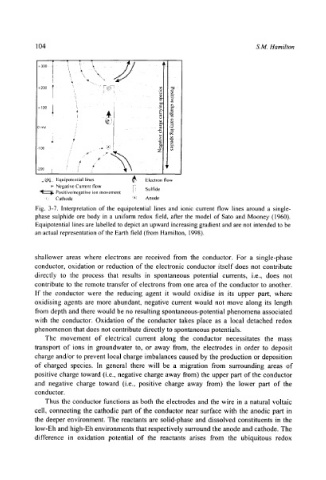
Fig. 3-7. Interpretation of the equipotential lines and ionic current flow lines around a single-
phase sulphide ore body in a uniform redox field, after the model of Sato and Mooney (1960).
Equipotential lines are labelled to depict an upward increasing gradient and are not intended to be
an actual representation of the Earth field (from Hamilton, 1998).
shallower areas where electrons are received from the conductor. For a single-phase
conductor, oxidation or reduction of the electronic conductor itself does not contribute
directly to the process that results in spontaneous potential currents, i.e., does not
contribute to the remote transfer of electrons from one area of the conductor to another.
If the conductor were the reducing agent it would oxidise in its upper part, where
oxidising agents are more abundant, negative current would not move along its length
from depth and there would be no resulting spontaneous-potential phenomena associated
with the conductor. Oxidation of the conductor takes place as a local detached redox
phenomenon that does not contribute directly to spontaneous potentials.
The movement of electrical current along the conductor necessitates the mass
transport of ions in groundwater to, or away from, the electrodes in order to deposit
charge and/or to prevent local charge imbalances caused by the production or deposition
of charged species. In general there will be a migration from surrounding areas of
positive charge toward (i.e., negative charge away from) the upper part of the conductor
and negative charge toward (i.e., positive charge away from) the lower part of the
conductor.
Thus the conductor functions as both the electrodes and the wire in a natural voltaic
cell, connecting the cathodic part of the conductor near surface with the anodic part in
the deeper environment. The reactants are solid-phase and dissolved constituents in the
low-Eh and high-Eh environments that respectively surround the anode and cathode. The
difference in oxidation potential of the reactants arises from the ubiquitous redox