Page 132 - Geochemical Remote Sensing of The Sub-Surface
P. 132
Spontaneous potentials and electrochemical cells 109
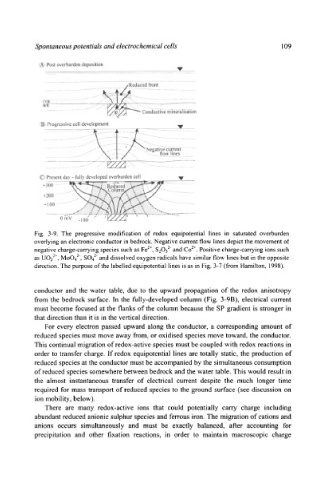
Fig. 3-9. The progressive modification of redox equipotential lines in saturated overburden
overlying an electronic conductor in bedrock. Negative current flow lines depict the movement of
negative charge-carrying species such as Fe 2+, 82032" and Co 2§ Positive charge-carrying ions such
as UO22§ MoO4 2, SO4 2 and dissolved oxygen radicals have similar flow lines but in the opposite
direction. The purpose of the labelled equipotential lines is as in Fig. 3-7 (from Hamilton, 1998).
conductor and the water table, due to the upward propagation of the redox anisotropy
from the bedrock surface. In the fully-developed column (Fig. 3-9B), electrical current
must become focused at the flanks of the column because the SP gradient is stronger in
that direction than it is in the vertical direction.
For every electron passed upward along the conductor, a corresponding amount of
reduced species must move away from, or oxidised species move toward, the conductor.
This continual migration of redox-active species must be coupled with redox reactions in
order to transfer charge. If redox equipotential lines are totally static, the production of
reduced species at the conductor must be accompanied by the simultaneous consumption
of reduced species somewhere between bedrock and the water table. This would result in
the almost instantaneous transfer of electrical current despite the much longer time
required for mass transport of reduced species to the ground surface (see discussion on
ion mobility, below).
There are many redox-active ions that could potentially carry charge including
abundant reduced anionic sulphur species and ferrous iron. The migration of cations and
anions occurs simultaneously and must be exactly balanced, after accounting for
precipitation and other fixation reactions, in order to maintain macroscopic charge