Page 129 - Geochemical Remote Sensing of The Sub-Surface
P. 129
106 S.M. Hamilton
+300 ................. ....... " ....... " -id
]
l
..... - ..... 9 ... ii ,,. ........
..... ~ --Negative Current ........................
Flow Lines .~. 3', ..... ........... O
~ .... -.. .... ,., 9 .0
~ -. "~..
Pyrite r
..,..." .. ~
+ ]ool- ............................................... ~ ....
0"Q
Fe S 1__~ 4FeS + 3Fe + 6e }"/" j''~Jr ,., " .... r t.~
, , . : .................... ~, _ .
r ,...,.
0 mV . . . . . . . . . . . . . . . . . . . . . . . Z
........ - ,,. ...............................
-]00
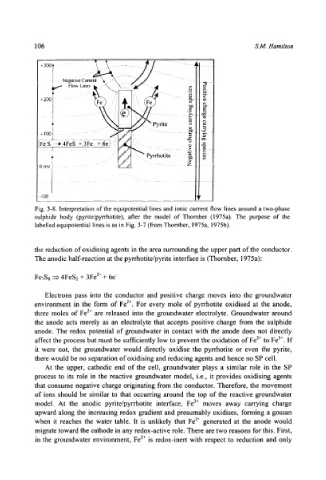
Fig. 3-8. Interpretation of the equipotential lines and ionic current flow lines around a two-phase
sulphide body (pyrite/pyrrhotite), after the model of Thornber (1975a). The purpose of the
labelled equipotential lines is as in Fig. 3-7 (from Thornber, 1975a, 1975b).
the reduction of oxidising agents in the area surrounding the upper part of the conductor.
The anodic half-reaction at the pyrrhotite/pyrite interface is (Thornber, 1975a):
FeTS8 ~ 4FeS/+ 3Fe 2+ + 6e-
Electrons pass into the conductor and positive charge moves into the groundwater
environment in the form of Fe 2§ For every mole of pyrrhotite oxidised at the anode,
three moles of Fe 2§ are released into the groundwater electrolyte. Groundwater around
the anode acts merely as an electrolyte that accepts positive charge from the sulphide
anode. The redox potential of groundwater in contact with the anode does not directly
affect the process but must be sufficiently low to prevent the oxidation of Fe 2§ to Fe 3§ If
it were not, the groundwater would directly oxidise the pyrrhotite or even the pyrite,
there would be no separation of oxidising and reducing agents and hence no SP cell.
At the upper, cathodic end of the cell, groundwater plays a similar role in the SP
process to its role in the reactive groundwater model, i.e., it provides oxidising agents
that consume negative charge originating from the conductor. Therefore, the movement
of ions should be similar to that occurring around the top of the reactive groundwater
model. At the anodic pyrite/pyrrhotite interface, Fe 2+ moves away carrying charge
upward along the increasing redox gradient and presumably oxidises, forming a gossan
when it reaches the water table. It is unlikely that Fe 2§ generated at the anode would
migrate toward the cathode in any redox-active role. There are two reasons for this. First,
in the groundwater environment, Fe 2§ is redox-inert with respect to reduction and only