Page 137 - Geochemical Remote Sensing of The Sub-Surface
P. 137
114 S.M. Hamilton
1 O0 H §
~ x-
~ ~0
"0
0 I
0 2000 4000 6000 800C
Time (yeors} for ton migrotion through overburden
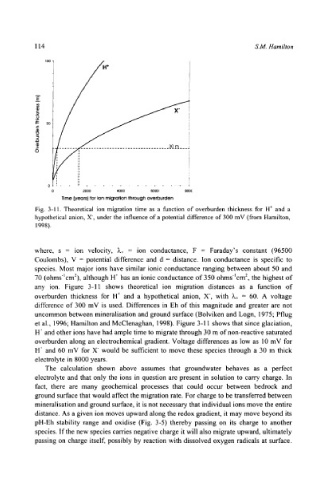
Fig. 3-11. Theoretical ion migration time as a function of overburden thickness for H + and a
hypothetical anion, X, under the influence of a potential difference of 300 mV (from Hamilton,
1998).
where, s = ion velocity, ~.+ = ion conductance, F = Faraday's constant (96500
Coulombs), V = potential difference and d = distance. Ion conductance is specific to
species. Most major ions have similar ionic conductance ranging between about 50 and
70 (ohms-lcm2), although H + has an ionic conductance of 350 ohms-lcm 2, the highest of
any ion. Figure 3-11 shows theoretical ion migration distances as a function of
overburden thickness for H § and a hypothetical anion, X-, with k+ = 60. A voltage
difference of 300 mV is used. Differences in Eh of this magnitude and greater are not
uncommon between mineralisation and ground surface (Bolviken and Logn, 1975; Pflug
et al., 1996; Hamilton and McClenaghan, 1998). Figure 3-11 shows that since glaciation,
H + and other ions have had ample time to migrate through 30 m of non-reactive saturated
overburden along an electrochemical gradient. Voltage differences as low as 10 mV for
H + and 60 mV for X would be sufficient to move these species through a 30 m thick
electrolyte in 8000 years.
The calculation shown above assumes that groundwater behaves as a perfect
electrolyte and that only the ions in question are present in solution to carry charge. In
fact, there are many geochemical processes that could occur between bedrock and
ground surface that would affect the migration rate. For charge to be transferred between
mineralisation and ground surface, it is not necessary that individual ions move the entire
distance. As a given ion moves upward along the redox gradient, it may move beyond its
pH-Eh stability range and oxidise (Fig. 3-5) thereby passing on its charge to another
species. If the new species carries negative charge it will also migrate upward, ultimately
passing on charge itself, possibly by reaction with dissolved oxygen radicals at surface.