Page 106 - Geochemistry of Oil Field Waters
P. 106
94 ANALYSIS OF OILFIELD WATERS
Iron
The spectrochemical procedure will give values only for total iron and will
not differentiate ferrous iron from ferric iron. The following procedure can
be used to determine F-+* and Fe+3 in a freshly sampled water (Collins et
al., 1961).
Reagents and apparatus. Standard iron solution: dissolve 1.00 g of
hydrogen-reduced iron in a minimum of hydrochloric acid and dilute to 1
liter with distilled water. This solution contains 1 mg/ml of iron. Transfer 10
ml of this solution to a l-liter flask and dilute to volume with distilled water.
1 ml of this solution contains 0.01 mg of iron.
Hydroquinone solution: dissolve 1 g of hydroquinone in 100 ml of distil-
led water.
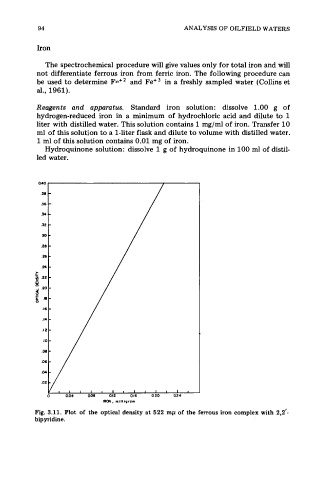
IRON, milligram
Fig. 3.11. Plot of the optical density at 522 mp of the ferrous iron complex with 2,2'-
bip yridine.