Page 132 - Geochemistry of Oil Field Waters
P. 132
120 ANALYSIS OF OILFIELD WATERS
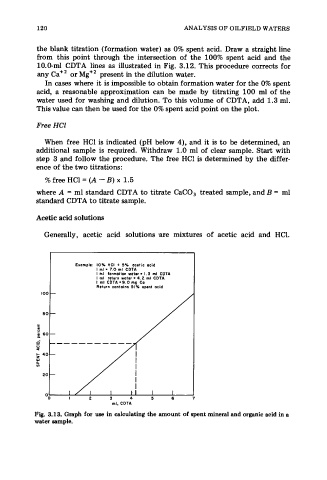
the blank titration (formation water) as 0% spent acid. Draw a straight line
from this point through the intersection of the 100% spent acid and the
10.0-ml CDTA lines as illustrated in Fig. 3.12. This procedure corrects for
any Ca+’ or Mg+2 present in the dilution water.
In cases where it is impossible to obtain formation water for the 0% spent
acid, a reasonable approximation can be made by titrating 100 ml of the
water used for washing and dilution. To this volume of CDTA, add 1.3 ml.
This value can then be used for the 0% spent acid point on the plot.
Free HCl
When free HC1 is indicated (pH below 4), and it is to be determined, an
additional sample is required. Withdraw 1.0 ml of clear sample. Start with
step 3 and follow the procedure. The free HC1 is determined by the differ-
ence of the two titrations:
% free HC1= (A -B) x 1.5
where A = ml standard CDTA to titrate CaCO, treated sample, and B = ml
standard CDTA to titrate sample.
Acetic acid solutions
Generally, acetic acid solutions are mixtures of acetic acid and HC1.
Example: 10% HCI t 5% acetic acid
I ml = 7.0 ml CDTA
I ml formation waler = 1.3 ml CDTA
I ml return water = 4.2 ml CDTA
I ml CDTA = 9.0 mg Ca
Return canlains 51% rpent acid
ml, CDTA
Fig. 3.13. Graph for use in calculating the amount of spent mineral and organic acid in a
water sample.