Page 23 - Geochemistry of Oil Field Waters
P. 23
12 SAMPLING SUBSURFACE OILFIELD WATERS
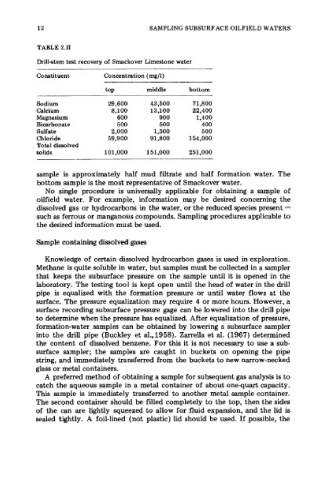
TABLE 2.11
Drill-stem test recovery of Smackover Limestone water
Constituent Concentration (mg/l)
top middle bottom
-
Sodium 29,600 43,500 71,800
Calcium 8,100 13,100 22,400
Magnesium 600 900 1,400
Bicarbonate 500 500 400
Sulfate 2,000 1,300 500
Chloride 59,900 91,800 154,000
Total dissolved
solids 101,000 151,000 251,000
sample is approximately half mud filtrate and half formation water. The
bottom sample is the most representative of Smackover water.
No single procedure is universally applicable for obtaining a sample of
oilfield water. For example, information may be desired concerning the
dissolved gas or hydrocarbons in the water, or the reduced species present -
such as ferrous or manganous compounds. Sampling procedures applicable to
the desired information must be used.
Sample containing dissolved gases
Knowledge of certain dissolved hydrocarbon gases is used in exploration.
Methane is quite soluble in water, but samples must be collected in a sampler
that keeps the subsurface pressure on the sample until it is opened in the
laboratory. The testing tool is kept open until the head of water in the drill
pipe is equalized with the formation pressure or until water flows at the
surface. The pressure equalization may require 4 or more hours. However, a
surface recording subsurface pressure gage can be lowered into the drill pipe
to determine when the pressure has equalized. After equalization of pressure,
formation-water samples can be obtained by lowering a subsurface sampler
into the drill pipe (Buckley et al.,1958). Zarrella et al. (1967) determined
the content of dissolved benzene. For this it is not necessary to use a sub-
surface sampler; the samples are caught in buckets on opening the pipe
string, and immediately transferred from the buckets to new narrow-necked
glass or metal containers.
A preferred method of obtaining a sample for subsequent gas analysis is to
catch the aqueous sample in a metal container of about one-quart capacity.
This sample is immediately transferred to another metal sample container.
The second container should be filled completely to the top, then the sides
of the can are lightly squeezed to allow for fluid expansion, and the lid is
sealed tightly. A foil-lined (not plastic) lid should be used. If possible, the