Page 26 - Geochemistry of Oil Field Waters
P. 26
SAMPLE FOR STABLE-ISOTOPE ANALYSIS 15
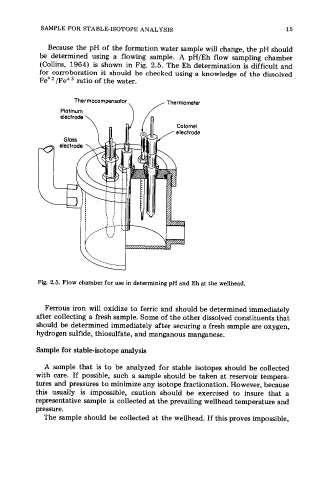
Because the pH of the formation water sample will change, the pH should
be determined using a flowing sample. A pH/Eh flow sampling chamber
(Collins, 1964) is shown in Fig. 2.5. The Eh determination is difficult and
for corroboration it should be checked using a knowledge of the dissolved
Fe+* /Fe+3 ratio of the water.
Ther mocompensator
Ther
mocompensator
Fig. 2.5. Flow chamber for use in determining pH and Eh at the wellhead.
Ferrous iron will oxidize to ferric and should be determined immediately
after collecting a fresh sample. Some of the other dissolved constituents that
should be determined immediately after securing a fresh sample are oxygen,
hydrogen sulfide, thiosulfate, and manganous manganese.
Sample for stable-isotope analysis
A sample that is to be analyzed for stable isotopes should be collected
with care. If possible, such a sample should be taken at reservoir tempera-
tures and pressures to minimize any isotope fractionation. However, because
this usually is impossible, caution should be exercised to insure that a
representative sample is collected at the prevailing wellhead temperature and
pressure.
The sample should be collected at the wellhead. If this proves impossible,