Page 273 - Geothermal Energy Renewable Energy and The Environment
P. 273
264 Geothermal Energy: Renewable Energy and the Environment
Temp.°C
200
150
100
0
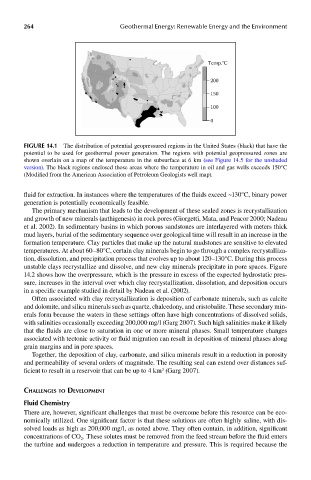
FIGUre 14.1 The distribution of potential geopressured regions in the United States (black) that have the
potential to be used for geothermal power generation. The regions with potential geopressured zones are
shown overlain on a map of the temperature in the subsurface at 6 km (see Figure 14.5 for the unshaded
version). The black regions enclosed those areas where the temperature in oil and gas wells exceeds 150°C
(Modified from the American Association of Petroleum Geologists well map).
fluid for extraction. In instances where the temperatures of the fluids exceed ~130°C, binary power
generation is potentially economically feasible.
The primary mechanism that leads to the development of these sealed zones is recrystallization
and growth of new minerals (authigenesis) in rock pores (Giorgetti, Mata, and Peacor 2000; Nadeau
et al. 2002). In sedimentary basins in which porous sandstones are interlayered with meters thick
mud layers, burial of the sedimentary sequence over geological time will result in an increase in the
formation temperature. Clay particles that make up the natural mudstones are sensitive to elevated
temperatures. At about 60–80°C, certain clay minerals begin to go through a complex recrystalliza-
tion, dissolution, and precipitation process that evolves up to about 120–130°C. During this process
unstable clays recrystallize and dissolve, and new clay minerals precipitate in pore spaces. Figure
14.2 shows how the overpressure, which is the pressure in excess of the expected hydrostatic pres-
sure, increases in the interval over which clay recrystallization, dissolution, and deposition occurs
in a specific example studied in detail by Nadeau et al. (2002).
Often associated with clay recrystallization is deposition of carbonate minerals, such as calcite
and dolomite, and silica minerals such as quartz, chalcedony, and cristobalite. These secondary min-
erals form because the waters in these settings often have high concentrations of dissolved solids,
with salinities occasionally exceeding 200,000 mg/l (Garg 2007). Such high salinities make it likely
that the fluids are close to saturation in one or more mineral phases. Small temperature changes
associated with tectonic activity or fluid migration can result in deposition of mineral phases along
grain margins and in pore spaces.
Together, the deposition of clay, carbonate, and silica minerals result in a reduction in porosity
and permeability of several orders of magnitude. The resulting seal can extend over distances suf-
3
ficient to result in a reservoir that can be up to 4 km (Garg 2007).
challenGes To developmenT
Fluid chemistry
There are, however, significant challenges that must be overcome before this resource can be eco-
nomically utilized. One significant factor is that these solutions are often highly saline, with dis-
solved loads as high as 200,000 mg/l, as noted above. They often contain, in addition, significant
concentrations of CO . These solutes must be removed from the feed stream before the fluid enters
2
the turbine and undergoes a reduction in temperature and pressure. This is required because the