Page 274 - Geothermal Energy Renewable Energy and The Environment
P. 274
The Geothermal Energy Future: Possibilities and Issues 265
1800
2000
2200
Depth (m) 2400 Clay mineral
authigenesis
2600
2800
0 0.689 1.379 2.068
Over pressure MPa
FIGUre 14.2 Overpressure (measured pressure-hydrostatic pressure, in MPa), as a function of depth in a
geopressured well on the Norwegian continental shelf. The increase in overpressure in the depth interval from
2400 m to 2700 m corresponds to the interval over which clay mineralogy is evolving. (Nadeau, P. H., Peacor,
D. R., Yan, J., and Hillier, S., American Mineralogist, 87:1580–89, 2002.)
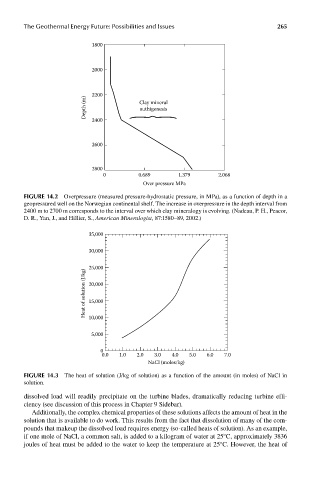
35,000
30,000
Heat of solution (J/kg) 25,000
20,000
15,000
10,000
5,000
0
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0
NaCl (moles/kg)
FIGUre 14.3 The heat of solution (J/kg of solution) as a function of the amount (in moles) of NaCl in
solution.
dissolved load will readily precipitate on the turbine blades, dramatically reducing turbine effi-
ciency (see discussion of this process in Chapter 9 Sidebar).
Additionally, the complex chemical properties of these solutions affects the amount of heat in the
solution that is available to do work. This results from the fact that dissolution of many of the com-
pounds that makeup the dissolved load requires energy (so-called heats of solution). As an example,
if one mole of NaCl, a common salt, is added to a kilogram of water at 25°C, approximately 3836
joules of heat must be added to the water to keep the temperature at 25°C. However, the heat of