Page 121 - Geothermal Energy Systems Exploration, Development, and Utilization
P. 121
2.5 Geochemistry 97
The isotopic ratios of both nonfractionating and fractionating elements in
groundwater at any point along a fluid flow path will be the product of the iso-
topic composition of the original fluid and that of the solute acquired from the
host rock. Therefore, isotope ratio measurements, either on sampled groundwa-
ter or water-deposited minerals, yield spatial or temporal patterns that contain
information concerning groundwater flow and transport conditions.
Many geochemical techniques, more or less sophisticated, are effective to indicate
the presence of geothermal reservoirs at depth and can be used to map their
extension (Chiodini et al., 1998).
2.5.7
Estimation of Reservoir Temperature
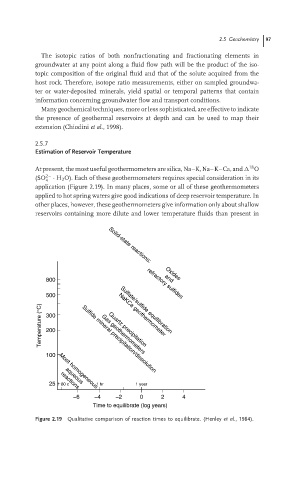
18
At present, the most useful geothermometers are silica, Na–K, Na–K–Ca, and O
(SO 2– · H 2 O). Each of these geothermometers requires special consideration in its
4
application (Figure 2.19). In many places, some or all of these geothermometers
applied to hot spring waters give good indications of deep reservoir temperature. In
other places, however, these geothermometers give information only about shallow
reservoirs containing more dilute and lower temperature fluids than present in
Solid-state reactions:
Oxides
800 refractory sulfides
and
500 Sulfate/sulfide equilibration
Temperature (°C) 300 Sulfide mineral precipitation/dissolution
200
NaKCa geothermometer
Quartz precipitation
Gas geothermometers
100
aqueous
25 1 80 c 1 hr 1 year
Most homogeneous
reactions
−6 −4 −2 0 2 4
Time to equilibrate (log years)
Figure 2.19 Qualitative comparison of reaction times to equilibrate. (Henley et al., 1984).