Page 119 - Geothermal Energy Systems Exploration, Development, and Utilization
P. 119
2.5 Geochemistry 95
the range may reflect variations in the contributions from different heat sources
(Nicholson, 1993).
The very low solubility of noble gases in water make them very sensitive
natural tracers for monitoring the return of cooler reinjected production fluids in
geothermal reservoirs. Field management and production strategies need a reliable
and sensitive tracer for monitoring the breakthrough of reinjected fluids (D’Amore
and Panichi, 1987).
Fractionating elements (H, C, O, and N) are elements whose isotopic composition
can be modified by chemical reactions involving the breakdown of chemical bonds,
electron transfer, or by phase change (e.g., boiling and condensation), (Horita and
Wesolowski, 1994). These elements can provide useful information regarding the
source of recharge (meteoric) fluids, water/rock ratios, and chemical equilibration
temperatures.
18
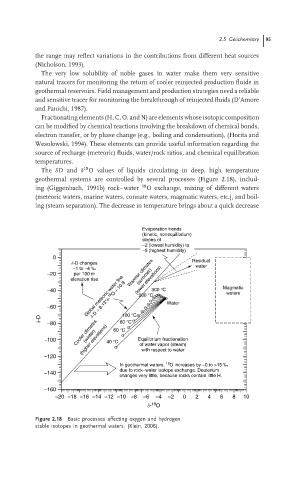
The δDand δ O values of liquids circulating in deep, high temperature
geothermal systems are controlled by several processes (Figure 2.18), includ-
ing (Giggenbach, 1991b) rock–water 18 O exchange, mixing of different waters
(meteoric waters, marine waters, connate waters, magmatic waters, etc.), and boil-
ing (steam separation). The decrease in temperature brings about a quick decrease
Evaporation trends
(kinetic, nonequilibrium)
slopes of
~2 (lowest humidity) to
~5 (highest humidity)
0
d-D changes Residual
−1 to −4 ‰ water
(summer)
−20 per 100 m Warmer climates
Global meteoric water line 200 °C
elevation rise (lower elevations)
d-D = 8.13*d- 18 O + 10.8
−40 300 °C Magmatic
waters
−60 Water
100 °C
d-D −80 80 °C
Cooler climates 60 °C
(winter)
−100 (higher elevations) 40 °C Equilibrium fractionation
of water vapor (steam)
−120 with respect to water
18
In geothermal waters, O increases by ~0 to >15 ‰
due to rock–water isotope exchange. Deuterium
−140
changes very little, because rocks contain little H.
−160
−20 −18 −16 −14 −12 −10 −8 −6 −4 −2 0 2 4 6 8 10
18
d- O
Figure 2.18 Basic processes affecting oxygen and hydrogen
stable isotopes in geothermal waters. (Klein, 2006).