Page 115 - Geothermal Energy Systems Exploration, Development, and Utilization
P. 115
2.5 Geochemistry 91
2.5.5
Chemical Characteristics of Fluids
The chemical classification of waters is essential for a correct utilization of geochem-
ical techniques, which can be confidently applied only to particular kinds of fluids
with limited ranges of composition, reflecting the environment of provenance. For
instance, most ionic solute geothermometers can be applied only to the samples
representative of water–rock equilibrium at depth. Therefore, these samples have
to be properly identified and selected. Furthermore, possible phenomena affecting
the original characteristics of waters (i.e., addition of cold, shallow groundwaters,
boiling, dissolution, or precipitation of mineral phases) have to be recognized and
evaluated.
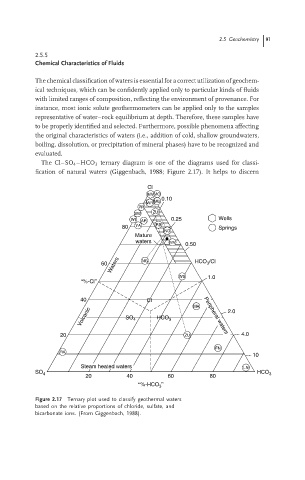
The Cl–SO 4 –HCO 3 ternary diagram is one of the diagrams used for classi-
fication of natural waters (Giggenbach, 1988; Figure 2.17). It helps to discern
Cl
MV MO
0.10
WR MU
PR
SW ZU
WI AR 0.25 Wells
80 YA RB Springs
NG
Mature
waters MV
0.50
Waters
60 NG HCO 3 /Cl
WS 1.0
‘‘%-Cl’’
40 Cl MA
Volcanic SO 4 HCO 3 Peripheral waters 2.0
20 ZU 4.0
FN
RA
10
Steam heated waters LN
SO 4 HCO 3
20 40 60 80
‘‘%-HCO 3 ’’
Figure 2.17 Ternary plot used to classify geothermal waters
based on the relative proportions of chloride, sulfate, and
bicarbonate ions. (From Giggenbach, 1988).