Page 114 - Geothermal Energy Systems Exploration, Development, and Utilization
P. 114
90 2 Exploration Methods
2800
Steam
2000
Enthalpy (J g −1 ) Aquifer fluid (265 °C)
1000
Cold water mixing Steam loss
Aquifer fluid
flashed to
100 °C
1000 2000
−1
Chloride (mg kg )
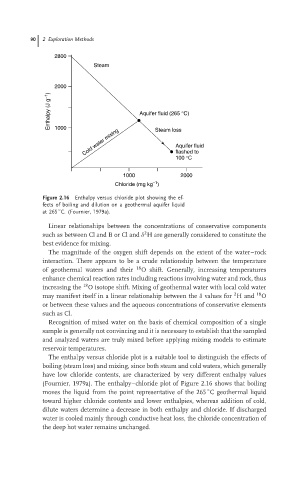
Figure 2.16 Enthalpy versus chloride plot showing the ef-
fects of boiling and dilution on a geothermal aquifer liquid
at 265 C. (Fournier, 1979a).
◦
Linear relationships between the concentrations of conservative components
2
such as between Cl and B or Cl and δ H are generally considered to constitute the
best evidence for mixing.
The magnitude of the oxygen shift depends on the extent of the water–rock
interaction. There appears to be a crude relationship between the temperature
of geothermal waters and their 18 O shift. Generally, increasing temperatures
enhance chemical reaction rates including reactions involving water and rock, thus
increasing the 18 O isotope shift. Mixing of geothermal water with local cold water
2
may manifest itself in a linear relationship between the δ values for Hand 18 O
or between these values and the aqueous concentrations of conservative elements
such as Cl.
Recognition of mixed water on the basis of chemical composition of a single
sample is generally not convincing and it is necessary to establish that the sampled
and analyzed waters are truly mixed before applying mixing models to estimate
reservoir temperatures.
The enthalpy versus chloride plot is a suitable tool to distinguish the effects of
boiling (steam loss) and mixing, since both steam and cold waters, which generally
have low chloride contents, are characterized by very different enthalpy values
(Fournier, 1979a). The enthalpy–chloride plot of Figure 2.16 shows that boiling
◦
moves the liquid from the point representative of the 265 C geothermal liquid
toward higher chloride contents and lower enthalpies, whereas addition of cold,
dilute waters determine a decrease in both enthalpy and chloride. If discharged
water is cooled mainly through conductive heat loss, the chloride concentration of
the deep hot water remains unchanged.