Page 146 - Glucose Monitoring Devices
P. 146
147
Available CGM systems
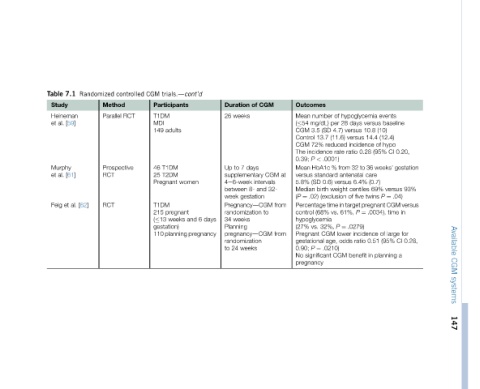
events baseline (10) (12.4) hypo of 0.20, CI (95% from 32 to 36 weeks’ gestation (0.7) 93% versus 69% .04) ¼ P in time .0034), for large of 0.28, CI (95% 0.51 a planning
hypoglycemia versus days 10.8 versus 14.4 versus incidence 0.28 ratio care antenatal 6.4% centiles twins five of Percentage time in target pregnant CGM versus ¼ P .0279) incidence ratio in benefit
of 28 per 4.7) (SD (11.6) reduced rate .0001) % standard versus 0.6) weight (exclusion 61%, vs. ¼ P 32%, lower CGM odds age, .0210) CGM
Outcomes number Mean mg/dL) ( 54 3.5 CGM 13.7 Control 72% CGM incidence The < P 0.39; HbA1c Mean versus (SD 5.8% birth Median .02) ¼ (P (68% control hypoglycemia vs. (27% Pregnant gestational ¼ P 0.90; significant No pregnancy
at from from
CGM CGM intervals 32- and to
of days 8- gestation PregnancydCGM pregnancydCGM weeks
Duration weeks 26 7 to Up supplementary 4e6-week between week randomization weeks 34 Planning randomization 24 to
days 6
trials.dcont’d Participants adults T1DM T2DM women pregnant and weeks 110 planning pregnancy
CGM T1DM MDI 149 46 25 Pregnant T1DM 215 ( 13 gestation)
controlled Method RCT Prospective
Randomized Parallel RCT RCT [62]
7.1 Heineman [59] [61] al. et
Table Study al. et Murphy al. et Feig