Page 255 - Glucose Monitoring Devices
P. 255
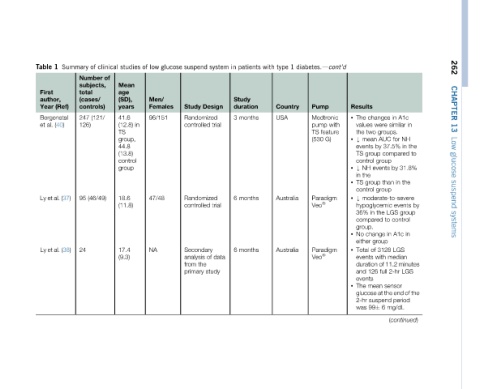
262 CHAPTER 13 Low glucose suspend systems
the to by
in 31.8% group in minutes LGS
A1c NH in the events control LGS period (continued)
in similar groups. for 37.5% compared by in than moderate-to-severe LGS to A1c in median of 11.2 2-hr sensor glucose at the end of the mg/dL
changes were two AUC by group group events group group hypoglycemic the in change group 3128 of with full 126 mean suspend 6 99
Results The • values the mean Y • events TS control NH Y • the in TS • control Y • 36% compared group. No • either Total • events duration and events The • 2-hr was
diabetes.dcont’d Pump Medtronic with pump feature TS G) (530 Paradigm Veo Ò Paradigm Veo Ò
1 Country
type USA Australia Australia
with
patients Study duration months months months
in 3 6 6
system Design trial trial data of study
suspend Study Randomized controlled Randomized controlled Secondary analysis the from primary
glucose
low Men/ Females 96/151 47/48 NA
of
studies Mean age (SD), years 41.6 in (12.8) TS group, 44.8 (13.8) control group 18.6 (11.8) 17.4 (9.3)
clinical of
of Number subjects, total (cases/ controls) (121/ 247 126) (46/49)
Summary 95 24
1 author, (Ref) Bergenstal (40) Ly et al. (37) Ly et al. (38)
Table First Year al. et