Page 243 - Handbook of Thermal Analysis of Construction Materials
P. 243
Section 2.0 - Lignosulfonates 225
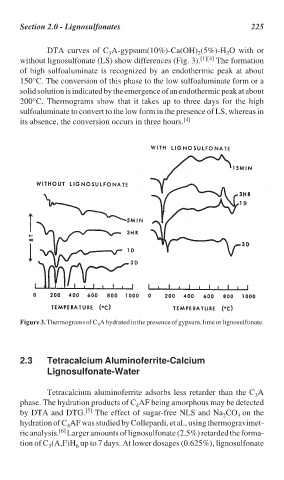
DTA curves of C A-gypsum(10%)-Ca(OH) (5%)-H O with or
3
2
2
without lignosulfonate (LS) show differences (Fig. 3). [1][4] The formation
of high sulfoaluminate is recognized by an endothermic peak at about
150°C. The conversion of this phase to the low sulfoaluminate form or a
solid solution is indicated by the emergence of an endothermic peak at about
200°C. Thermograms show that it takes up to three days for the high
sulfoaluminate to convert to the low form in the presence of LS, whereas in
its absence, the conversion occurs in three hours. [4]
Figure 3. Thermograms of C A hydrated in the presence of gypsum, lime or lignosulfonate.
3
2.3 Tetracalcium Aluminoferrite-Calcium
Lignosulfonate-Water
Tetracalcium aluminoferrite adsorbs less retarder than the C A
3
phase. The hydration products of C AF being amorphous may be detected
4
[5]
by DTA and DTG. The effect of sugar-free NLS and Na CO on the
2
3
hydration of C AF was studied by Collepardi, et al., using thermogravimet-
4
[6]
ric analysis. Larger amounts of lignosulfonate (2.5%) retarded the forma-
tion of C (A,F)H up to 7 days. At lower dosages (0.625%), lignosulfonate
3
6