Page 245 - Handbook of Thermal Analysis of Construction Materials
P. 245
Section 2.0 - Lignosulfonates 227
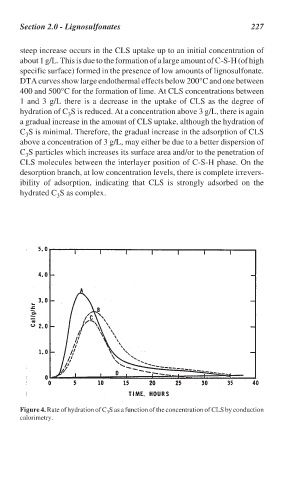
steep increase occurs in the CLS uptake up to an initial concentration of
about 1 g/L. This is due to the formation of a large amount of C-S-H (of high
specific surface) formed in the presence of low amounts of lignosulfonate.
DTA curves show large endothermal effects below 200°C and one between
400 and 500°C for the formation of lime. At CLS concentrations between
1 and 3 g/L there is a decrease in the uptake of CLS as the degree of
hydration of C S is reduced. At a concentration above 3 g/L, there is again
3
a gradual increase in the amount of CLS uptake, although the hydration of
C S is minimal. Therefore, the gradual increase in the adsorption of CLS
3
above a concentration of 3 g/L, may either be due to a better dispersion of
C S particles which increases its surface area and/or to the penetration of
3
CLS molecules between the interlayer position of C-S-H phase. On the
desorption branch, at low concentration levels, there is complete irrevers-
ibility of adsorption, indicating that CLS is strongly adsorbed on the
hydrated C S as complex.
3
Figure 4. Rate of hydration of C S as a function of the concentration of CLS by conduction
3
calorimetry.