Page 305 - Handbook of Thermal Analysis of Construction Materials
P. 305
282 Chapter 7 - Superplasticizing Admixtures
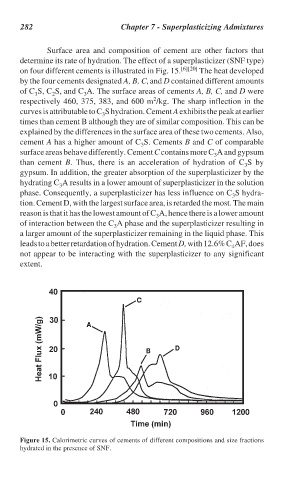
Surface area and composition of cement are other factors that
determine its rate of hydration. The effect of a superplasticizer (SNF type)
on four different cements is illustrated in Fig. 15. [6][20] The heat developed
by the four cements designated A, B, C, and D contained different amounts
of C S, C S, and C A. The surface areas of cements A, B, C, and D were
3
3
2
2
respectively 460, 375, 383, and 600 m /kg. The sharp inflection in the
curves is attributable to C S hydration. Cement A exhibits the peak at earlier
3
times than cement B although they are of similar composition. This can be
explained by the differences in the surface area of these two cements. Also,
cement A has a higher amount of C S. Cements B and C of comparable
3
surface areas behave differently. Cement C contains more C A and gypsum
3
than cement B. Thus, there is an acceleration of hydration of C S by
3
gypsum. In addition, the greater absorption of the superplasticizer by the
hydrating C A results in a lower amount of superplasticizer in the solution
3
phase. Consequently, a superplasticizer has less influence on C S hydra-
3
tion. Cement D, with the largest surface area, is retarded the most. The main
reason is that it has the lowest amount of C A, hence there is a lower amount
3
of interaction between the C A phase and the superplasticizer resulting in
3
a larger amount of the superplasticizer remaining in the liquid phase. This
leads to a better retardation of hydration. Cement D, with 12.6% C AF, does
4
not appear to be interacting with the superplasticizer to any significant
extent.
Figure 15. Calorimetric curves of cements of different compositions and size fractions
hydrated in the presence of SNF.