Page 350 - Handbook of Thermal Analysis of Construction Materials
P. 350
Section 6.0 - Metakaolinite 327
polymer formation which is responsible for strength development. A
shoulder also appears corresponding to a zeolite crystallization process. [67]
In DTA, an endothermal peak appearing at 165–175°C is attributed to the
process of dehydroxylation of the amorphous and crystalline zeolite. [68]
At ambient temperatures, MK is known to react with Ca, Na and K
hydroxides, and hydrated cement. Attempts have been made to increase the
rate of strength development by autoclaving mixtures of cement-quartz or
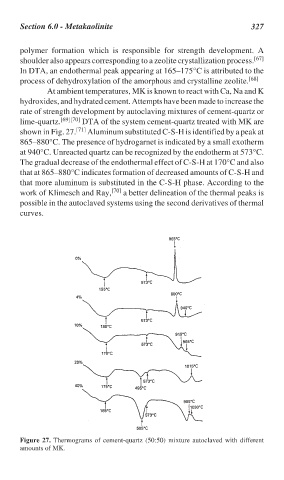
lime-quartz. [69][70] DTA of the system cement-quartz treated with MK are
shown in Fig. 27. [71] Aluminum substituted C-S-H is identified by a peak at
865–880°C. The presence of hydrogarnet is indicated by a small exotherm
at 940°C. Unreacted quartz can be recognized by the endotherm at 573°C.
The gradual decrease of the endothermal effect of C-S-H at 170°C and also
that at 865–880°C indicates formation of decreased amounts of C-S-H and
that more aluminum is substituted in the C-S-H phase. According to the
work of Klimesch and Ray, [70] a better delineation of the thermal peaks is
possible in the autoclaved systems using the second derivatives of thermal
curves.
Figure 27. Thermograms of cement-quartz (50:50) mixture autoclaved with different
amounts of MK.