Page 427 - Handbook of Thermal Analysis of Construction Materials
P. 427
404 Chapter 10 - Non-Portland Rapid Setting Cements
2.0 CALCIUM ALUMINATE CEMENTS
2.1 Basic Reactions
Calcium aluminate cements (CAC) have a wide range of alumina
content (38 to 90%). The chemistry and principle cement-water reactions
for high alumina cement—a widely used non-portland cement—are de-
scribed in detail in Ch. 9. The primary binding phase is calcium
monoaluminate (CaAl O or CA). Refractory cements contain higher
2 4
alumina contents (70 to 90%).
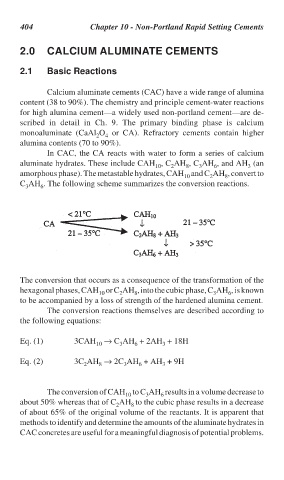
In CAC, the CA reacts with water to form a series of calcium
aluminate hydrates. These include CAH , C AH , C AH , and AH (an
8
2
10
3
3
6
amorphous phase). The metastable hydrates, CAH and C AH , convert to
10 2 8
C AH . The following scheme summarizes the conversion reactions.
6
3
The conversion that occurs as a consequence of the transformation of the
hexagonal phases, CAH or C AH , into the cubic phase, C AH , is known
10 2 8 3 6
to be accompanied by a loss of strength of the hardened alumina cement.
The conversion reactions themselves are described according to
the following equations:
Eq. (1) 3CAH → C AH + 2AH + 18H
3
6
3
10
Eq. (2) 3C AH → 2C AH + AH + 9H
6
3
8
3
2
The conversion of CAH to C AH results in a volume decrease to
10 3 6
about 50% whereas that of C AH to the cubic phase results in a decrease
2
8
of about 65% of the original volume of the reactants. It is apparent that
methods to identify and determine the amounts of the aluminate hydrates in
CAC concretes are useful for a meaningful diagnosis of potential problems.