Page 457 - Handbook of Thermal Analysis of Construction Materials
P. 457
432 Chapter 10 - Non-Portland Rapid Setting Cements
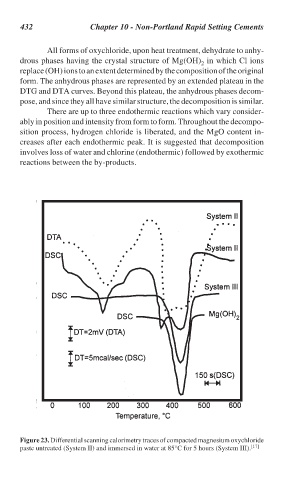
All forms of oxychloride, upon heat treatment, dehydrate to anhy-
drous phases having the crystal structure of Mg(OH) in which Cl ions
2
replace (OH) ions to an extent determined by the composition of the original
form. The anhydrous phases are represented by an extended plateau in the
DTG and DTA curves. Beyond this plateau, the anhydrous phases decom-
pose, and since they all have similar structure, the decomposition is similar.
There are up to three endothermic reactions which vary consider-
ably in position and intensity from form to form. Throughout the decompo-
sition process, hydrogen chloride is liberated, and the MgO content in-
creases after each endothermic peak. It is suggested that decomposition
involves loss of water and chlorine (endothermic) followed by exothermic
reactions between the by-products.
Figure 23. Differential scanning calorimetry traces of compacted magnesium oxychloride
paste untreated (System II) and immersed in water at 85°C for 5 hours (System III). [17]