Page 461 - Handbook of Thermal Analysis of Construction Materials
P. 461
436 Chapter 10 - Non-Portland Rapid Setting Cements
It is, therefore, apparent that differential thermal analysis (in
addition to chemical analysis) could be very useful in determining the
suitability of magnesites for making oxychloride cement.
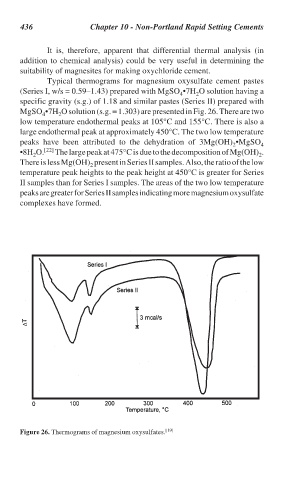
Typical thermograms for magnesium oxysulfate cement pastes
(Series I, w/s = 0.59–1.43) prepared with MgSO •7H O solution having a
4
2
specific gravity (s.g.) of 1.18 and similar pastes (Series II) prepared with
MgSO •7H O solution (s.g. = 1.303) are presented in Fig. 26. There are two
4
2
low temperature endothermal peaks at 105°C and 155°C. There is also a
large endothermal peak at approximately 450°C. The two low temperature
peaks have been attributed to the dehydration of 3Mg(OH) •MgSO 4
2
•8H O. [22] The large peak at 475°C is due to the decomposition of Mg(OH) .
2
2
There is less Mg(OH) present in Series II samples. Also, the ratio of the low
2
temperature peak heights to the peak height at 450°C is greater for Series
II samples than for Series I samples. The areas of the two low temperature
peaks are greater for Series II samples indicating more magnesium oxysulfate
complexes have formed.
Figure 26. Thermograms of magnesium oxysulfates. [19]