Page 486 - Handbook of Thermal Analysis of Construction Materials
P. 486
460 Chapter 11 - Gypsum and Gypsum Products
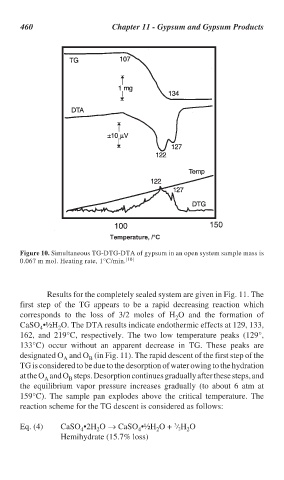
Figure 10. Simultaneous TG-DTG-DTA of gypsum in an open system sample mass is
0.067 m mol. Heating rate, 1°C/min. [10]
Results for the completely sealed system are given in Fig. 11. The
first step of the TG appears to be a rapid decreasing reaction which
corresponds to the loss of 3/2 moles of H O and the formation of
2
CaSO •½H O. The DTA results indicate endothermic effects at 129, 133,
4 2
162, and 219°C, respectively. The two low temperature peaks (129°,
133°C) occur without an apparent decrease in TG. These peaks are
designated O and O (in Fig. 11). The rapid descent of the first step of the
B
A
TG is considered to be due to the desorption of water owing to the hydration
at the O and O steps. Desorption continues gradually after these steps, and
B
A
the equilibrium vapor pressure increases gradually (to about 6 atm at
159°C). The sample pan explodes above the critical temperature. The
reaction scheme for the TG descent is considered as follows:
3
Eq. (4) CaSO •2H O → CaSO •½H O + / H O
4 2 4 2 2 2
Hemihydrate (15.7% loss)