Page 487 - Handbook of Thermal Analysis of Construction Materials
P. 487
Section 5.0 - Simultaneous TG - DTG - DTA 461
Eq. (5) CaSO •½H O → CaSO + ½H O
2
4
2
4
Anhydrite (5.2% loss)
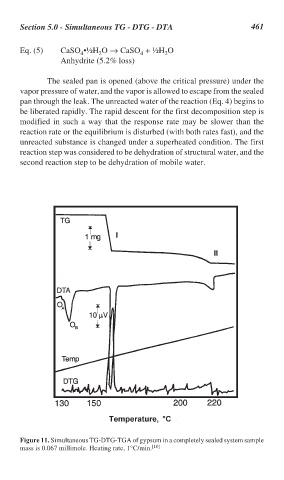
The sealed pan is opened (above the critical pressure) under the
vapor pressure of water, and the vapor is allowed to escape from the sealed
pan through the leak. The unreacted water of the reaction (Eq. 4) begins to
be liberated rapidly. The rapid descent for the first decomposition step is
modified in such a way that the response rate may be slower than the
reaction rate or the equilibrium is disturbed (with both rates fast), and the
unreacted substance is changed under a superheated condition. The first
reaction step was considered to be dehydration of structural water, and the
second reaction step to be dehydration of mobile water.
Figure 11. Simultaneous TG-DTG-TGA of gypsum in a completely sealed system sample
mass is 0.067 millimole. Heating rate, 1°C/min. [10]