Page 528 - Handbook of Thermal Analysis of Construction Materials
P. 528
Section 2.0 - Clays and Accessory Minerals 499
Clay Mineral Mixtures. Natural clay is generally made up of a
mixture of clay minerals. Some of the common clay minerals which occur in
nature are kaolinite, nontronite, illite, and montmorillonite. The endothermal
effects of kaolinite, illite, and nontronite occur in the range of 500–600°C,
so it would be difficult to identify them on the basis of the endothermal
effects. The exothermal crystallization at 900–1000°C shown by most clay
minerals is not easy to use to differentiate one clay mineral from the other.
It has been found that thermograms of kaolinite, illite, nontronite, and
montmorillonite complexed with malachite green or methylene blue give
characteristic exothermal peaks at 420, 460, 590, and 670°C respec-
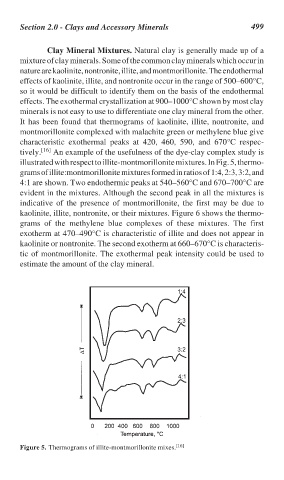
tively. [16] An example of the usefulness of the dye-clay complex study is
illustrated with respect to illite-montmorillonite mixtures. In Fig. 5, thermo-
grams of illite:montmorillonite mixtures formed in ratios of 1:4, 2:3, 3:2, and
4:1 are shown. Two endothermic peaks at 540–560°C and 670–700°C are
evident in the mixtures. Although the second peak in all the mixtures is
indicative of the presence of montmorillonite, the first may be due to
kaolinite, illite, nontronite, or their mixtures. Figure 6 shows the thermo-
grams of the methylene blue complexes of these mixtures. The first
exotherm at 470–490°C is characteristic of illite and does not appear in
kaolinite or nontronite. The second exotherm at 660–670°C is characteris-
tic of montmorillonite. The exothermal peak intensity could be used to
estimate the amount of the clay mineral.
Figure 5. Thermograms of illite-montmorillonite mixes. [16]