Page 57 - Handbook of Thermal Analysis of Construction Materials
P. 57
40 Chapter 2 - Introduction to Portland Cement Concrete
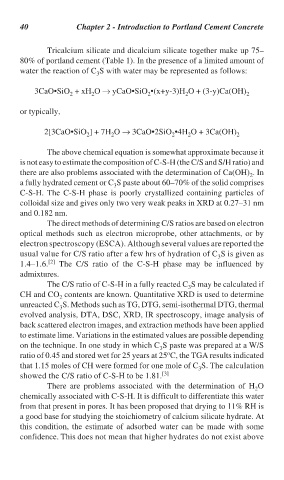
Tricalcium silicate and dicalcium silicate together make up 75–
80% of portland cement (Table 1). In the presence of a limited amount of
water the reaction of C S with water may be represented as follows:
3
3CaO•SiO + xH O → yCaO•SiO •(x+y-3)H O + (3-y)Ca(OH) 2
2
2
2
2
or typically,
2[3CaO•SiO ] + 7H O → 3CaO•2SiO •4H O + 3Ca(OH) 2
2
2
2
2
The above chemical equation is somewhat approximate because it
is not easy to estimate the composition of C-S-H (the C/S and S/H ratio) and
there are also problems associated with the determination of Ca(OH) . In
2
a fully hydrated cement or C S paste about 60–70% of the solid comprises
3
C-S-H. The C-S-H phase is poorly crystallized containing particles of
colloidal size and gives only two very weak peaks in XRD at 0.27–31 nm
and 0.182 nm.
The direct methods of determining C/S ratios are based on electron
optical methods such as electron microprobe, other attachments, or by
electron spectroscopy (ESCA). Although several values are reported the
usual value for C/S ratio after a few hrs of hydration of C S is given as
3
[2]
1.4–1.6. The C/S ratio of the C-S-H phase may be influenced by
admixtures.
The C/S ratio of C-S-H in a fully reacted C S may be calculated if
3
CH and CO contents are known. Quantitative XRD is used to determine
2
unreacted C S. Methods such as TG, DTG, semi-isothermal DTG, thermal
3
evolved analysis, DTA, DSC, XRD, IR spectroscopy, image analysis of
back scattered electron images, and extraction methods have been applied
to estimate lime. Variations in the estimated values are possible depending
on the technique. In one study in which C S paste was prepared at a W/S
3
o
ratio of 0.45 and stored wet for 25 years at 25 C, the TGA results indicated
that 1.15 moles of CH were formed for one mole of C S. The calculation
3
showed the C/S ratio of C-S-H to be 1.81. [3]
There are problems associated with the determination of H O
2
chemically associated with C-S-H. It is difficult to differentiate this water
from that present in pores. It has been proposed that drying to 11% RH is
a good base for studying the stoichiometry of calcium silicate hydrate. At
this condition, the estimate of adsorbed water can be made with some
confidence. This does not mean that higher hydrates do not exist above