Page 674 - Handbook of Thermal Analysis of Construction Materials
P. 674
642 Chapter 16 - Paints and Coatings
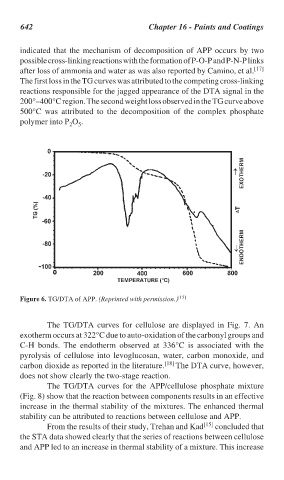
indicated that the mechanism of decomposition of APP occurs by two
possible cross-linking reactions with the formation of P-O-P and P-N-P links
after loss of ammonia and water as was also reported by Camino, et al. [17]
The first loss in the TG curves was attributed to the competing cross-linking
reactions responsible for the jagged appearance of the DTA signal in the
200°–400°C region. The second weight loss observed in the TG curve above
500°C was attributed to the decomposition of the complex phosphate
polymer into P O .
5
2
Figure 6. TG/DTA of APP. (Reprinted with permission.) [15]
The TG/DTA curves for cellulose are displayed in Fig. 7. An
exotherm occurs at 322°C due to auto-oxidation of the carbonyl groups and
C-H bonds. The endotherm observed at 336°C is associated with the
pyrolysis of cellulose into levoglucosan, water, carbon monoxide, and
carbon dioxide as reported in the literature. [18] The DTA curve, however,
does not show clearly the two-stage reaction.
The TG/DTA curves for the APP/cellulose phosphate mixture
(Fig. 8) show that the reaction between components results in an effective
increase in the thermal stability of the mixtures. The enhanced thermal
stability can be attributed to reactions between cellulose and APP.
From the results of their study, Trehan and Kad [15] concluded that
the STA data showed clearly that the series of reactions between cellulose
and APP led to an increase in thermal stability of a mixture. This increase