Page 100 - Geology and Geochemistry of Oil and Gas
P. 100
PHYSICAL AND CHEMICAL PROPERTIES OF WATERS 73
temperatures and pressures. It may be suggested that in some specific situation the
transition zone can actually extend over the entire thickness of the accumulation.
The following accumulations may be placed in this group:
(a) those where the OWC was determined from logs at the level of 50% oil
saturation (a quite frequent case); or
(b) those where the OWC was determined at the depth of perforated interval that
produced water-free oil, which is not rare.
In the latter case, one may also anticipate the effect of relative permeability. The
relative permeabilities to oil and to water depend on the rock properties (oil-wet
7
versus water-wet), alkalinity of water, and the presence of polar substances in oil.
The relative permeability to oil increases with increasing amount of polar substances
in the oil, whereas the relative permeability to water decreases (Langnes et al., 1972,
pp. 228–230). In the presence of alkaline water, the relative permeabilities both to oil
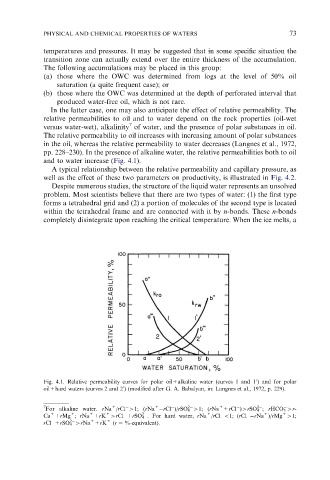
and to water increase (Fig. 4.1).
A typical relationship between the relative permeability and capillary pressure, as
well as the effect of these two parameters on productivity, is illustrated in Fig. 4.2.
Despite numerous studies, the structure of the liquid water represents an unsolved
problem. Most scientists believe that there are two types of water: (1) the first type
forms a tetrahedral grid and (2) a portion of molecules of the second type is located
within the tetrahedral frame and are connected with it by n-bonds. These n-bonds
completely disintegrate upon reaching the critical temperature. When the ice melts, a
0
Fig. 4.1. Relative permeability curves for polar oil+alkaline water (curves 1 and 1 ) and for polar
0
oil+hard waters (curves 2 and 2 ) (modified after G. A. Babalyan, in: Langnes et al., 1972, p. 229).
7 + + 2 + 2
For alkaline water. rNa /rCl 41; (rNa rCl )/rSO 4 41; (rNa +rCl )4rSO 4 ; rHCO 3 4r-
+
2
+
+
+
+
+
+
Ca +rMg ; rNa +rK 4rCl +rSO 4 . For hard water, rNa /rCl o1; (rCl rNa )/rMg 41;
+
2
rCl +rSO 4 4rNa +rK + (r ¼ %-equivalent).