Page 320 - Geology and Geochemistry of Oil and Gas
P. 320
WETTABILITY AND CAPILLARITY 281
Contaminants or impurities may exist in either fluid phase or may be adsorbed on
the solid surface. Even if present in minute quantities, they can and do change the
contact angle from the value measured for pure system (see Marsden, 1968).
A.3. EFFECT OF CONTACT ANGLE AND INTERFACIAL TENSION ON MOVEMENT OF OIL
For an ideal system composed of pure liquids, the advancing contact angle should
equal the receding angle. Because of the presence of impurities within the liquids,
however, the advancing contact angle is greater in most systems. The advancing
contact angle is the angle formed at the phase boundary when oil is displaced by
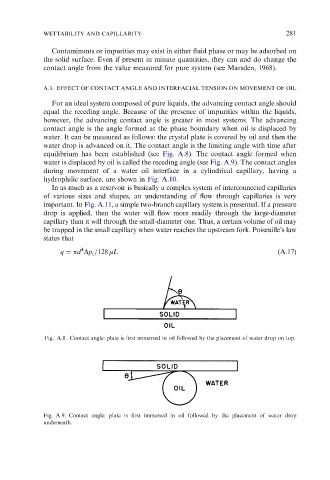
water. It can be measured as follows: the crystal plate is covered by oil and then the
water drop is advanced on it. The contact angle is the limiting angle with time after
equilibrium has been established (see Fig. A.8). The contact angle formed when
water is displaced by oil is called the receding angle (see Fig. A.9). The contact angles
during movement of a water–oil interface in a cylindrical capillary, having a
hydrophilic surface, are shown in Fig. A.10.
In as much as a reservoir is basically a complex system of interconnected capillaries
of various sizes and shapes, an understanding of flow through capillaries is very
important. In Fig. A.11, a simple two-branch capillary system is presented. If a pressure
drop is applied, then the water will flow more readily through the large-diameter
capillary than it will through the small-diameter one. Thus, a certain volume of oil may
be trapped in the small capillary when water reaches the upstream fork. Poiseuille’s law
states that
4
q ¼ pd Dp =128mL (A.17)
t
Fig. A.8. Contact angle: plate is first immersed in oil followed by the placement of water drop on top.
Fig. A.9. Contact angle: plate is first immersed in oil followed by the placement of water drop
underneath.