Page 315 - Geology and Geochemistry of Oil and Gas
P. 315
276 APPENDIX A
The wettability is calculated on the basis of core sample weights measured at various
testing modes. For example, the forced displacement of oil to S or and water to S iw may
be conducted using a centrifuge or by mounting the core in fluid-flow equipment and
pumping the displacing fluids into the core (see, e.g., Tiab and Donaldson, 2004, for
details, pp. 371–373).
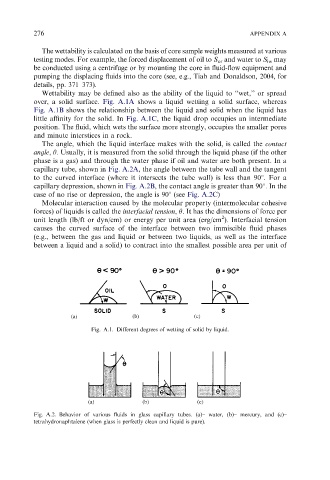
Wettability may be defined also as the ability of the liquid to ‘‘wet,’’ or spread
over, a solid surface. Fig. A.1A shows a liquid wetting a solid surface, whereas
Fig. A.1B shows the relationship between the liquid and solid when the liquid has
little affinity for the solid. In Fig. A.1C, the liquid drop occupies an intermediate
position. The fluid, which wets the surface more strongly, occupies the smaller pores
and minute interstices in a rock.
The angle, which the liquid interface makes with the solid, is called the contact
angle, y. Usually, it is measured from the solid through the liquid phase (if the other
phase is a gas) and through the water phase if oil and water are both present. In a
capillary tube, shown in Fig. A.2A, the angle between the tube wall and the tangent
to the curved interface (where it intersects the tube wall) is less than 901. For a
capillary depression, shown in Fig. A.2B, the contact angle is greater than 901. In the
case of no rise or depression, the angle is 901 (see Fig. A.2C)
Molecular interaction caused by the molecular property (intermolecular cohesive
forces) of liquids is called the interfacial tension, y. It has the dimensions of force per
2
unit length (lb/ft or dyn/cm) or energy per unit area (erg/cm ). Interfacial tension
causes the curved surface of the interface between two immiscible fluid phases
(e.g., between the gas and liquid or between two liquids, as well as the interface
between a liquid and a solid) to contract into the smallest possible area per unit of
Fig. A.1. Different degrees of wetting of solid by liquid.
Fig. A.2. Behavior of various fluids in glass capillary tubes. (a)– water, (b)– mercury, and (c)–
tetrahydronaphtalene (when glass is perfectly clean and liquid is pure).