Page 331 - Geology and Geochemistry of Oil and Gas
P. 331
292 APPENDIX B
0
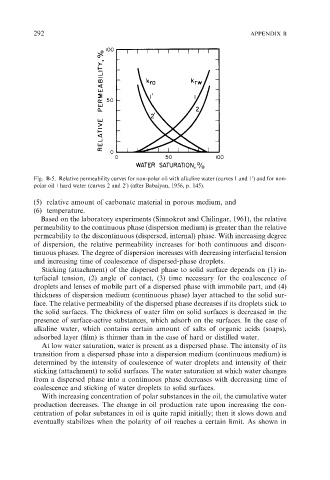
Fig. B-5. Relative permeability curves for non-polar oil with alkaline water (curves 1 and 1 ) and for non-
0
polar oil+hard water (curves 2 and 2 ) (after Babalyan, 1956, p. 145).
(5) relative amount of carbonate material in porous medium, and
(6) temperature.
Based on the laboratory experiments (Sinnokrot and Chilingar, 1961), the relative
permeability to the continuous phase (dispersion medium) is greater than the relative
permeability to the discontinuous (dispersed, internal) phase. With increasing degree
of dispersion, the relative permeability increases for both continuous and discon-
tinuous phases. The degree of dispersion increases with decreasing interfacial tension
and increasing time of coalescence of dispersed-phase droplets.
Sticking (attachment) of the dispersed phase to solid surface depends on (1) in-
terfacial tension, (2) angle of contact, (3) time necessary for the coalescence of
droplets and lenses of mobile part of a dispersed phase with immobile part, and (4)
thickness of dispersion medium (continuous phase) layer attached to the solid sur-
face. The relative permeability of the dispersed phase decreases if its droplets stick to
the solid surfaces. The thickness of water film on solid surfaces is decreased in the
presence of surface-active substances, which adsorb on the surfaces. In the case of
alkaline water, which contains certain amount of salts of organic acids (soaps),
adsorbed layer (film) is thinner than in the case of hard or distilled water.
At low water saturation, water is present as a dispersed phase. The intensity of its
transition from a dispersed phase into a dispersion medium (continuous medium) is
determined by the intensity of coalescence of water droplets and intensity of their
sticking (attachment) to solid surfaces. The water saturation at which water changes
from a dispersed phase into a continuous phase decreases with decreasing time of
coalescence and sticking of water droplets to solid surfaces.
With increasing concentration of polar substances in the oil, the cumulative water
production decreases. The change in oil production rate upon increasing the con-
centration of polar substances in oil is quite rapid initially; then it slows down and
eventually stabilizes when the polarity of oil reaches a certain limit. As shown in