Page 225 - Handbook Of Multiphase Flow Assurance
P. 225
224 10. Research methods in flow assurance
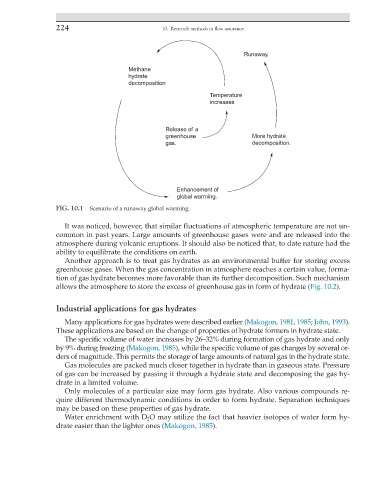
Runaway.
Methane
hydrate
decomposition
Temperature
increases
Release of a
greenhouse More hydrate
gas. decomposition.
Enhancement of
global warming.
FIG. 10.1 Scenario of a runaway global warming.
It was noticed, however, that similar fluctuations of atmospheric temperature are not un-
common in past years. Large amounts of greenhouse gases were and are released into the
atmosphere during volcanic eruptions. It should also be noticed that, to date nature had the
ability to equilibrate the conditions on earth.
Another approach is to treat gas hydrates as an environmental buffer for storing excess
greenhouse gases. When the gas concentration in atmosphere reaches a certain value, forma-
tion of gas hydrate becomes more favorable than its further decomposition. Such mechanism
allows the atmosphere to store the excess of greenhouse gas in form of hydrate (Fig. 10.2).
Industrial applications for gas hydrates
Many applications for gas hydrates were described earlier (Makogon, 1981, 1985; John, 1993).
These applications are based on the change of properties of hydrate formers in hydrate state.
The specific volume of water increases by 26–32% during formation of gas hydrate and only
by 9% during freezing (Makogon, 1985), while the specific volume of gas changes by several or-
ders of magnitude. This permits the storage of large amounts of natural gas in the hydrate state.
Gas molecules are packed much closer together in hydrate than in gaseous state. Pressure
of gas can be increased by passing it through a hydrate state and decomposing the gas hy-
drate in a limited volume.
Only molecules of a particular size may form gas hydrate. Also various compounds re-
quire different thermodynamic conditions in order to form hydrate. Separation techniques
may be based on these properties of gas hydrate.
Water enrichment with D 2 O may utilize the fact that heavier isotopes of water form hy-
drate easier than the lighter ones (Makogon, 1985).