Page 226 - Handbook Of Multiphase Flow Assurance
P. 226
Hydrate stability and crystal growth 225
Methane
hydrate
decomposition
Tempertature
increases
Release of a
greenhouse
gas.
No runaway.
Formation of
Increase of methane methane hydrate
concentration in to remove excess gas
atmosphere. from atmosphere.
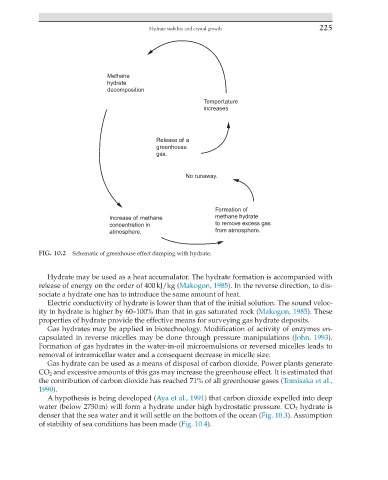
FIG. 10.2 Schematic of greenhouse effect damping with hydrate.
Hydrate may be used as a heat accumulator. The hydrate formation is accompanied with
release of energy on the order of 400 kJ/kg (Makogon, 1985). In the reverse direction, to dis-
sociate a hydrate one has to introduce the same amount of heat.
Electric conductivity of hydrate is lower than that of the initial solution. The sound veloc-
ity in hydrate is higher by 60–100% than that in gas saturated rock (Makogon, 1985). These
properties of hydrate provide the effective means for surveying gas hydrate deposits.
Gas hydrates may be applied in biotechnology. Modification of activity of enzymes en-
capsulated in reverse micelles may be done through pressure manipulations (John, 1993).
Formation of gas hydrates in the water-in-oil microemulsions or reversed micelles leads to
removal of intramicellar water and a consequent decrease in micelle size.
Gas hydrate can be used as a means of disposal of carbon dioxide. Power plants generate
CO 2 and excessive amounts of this gas may increase the greenhouse effect. It is estimated that
the contribution of carbon dioxide has reached 71% of all greenhouse gases (Tomisaka et al.,
1990).
A hypothesis is being developed (Aya et al., 1991) that carbon dioxide expelled into deep
water (below 2750 m) will form a hydrate under high hydrostatic pressure. CO 2 hydrate is
denser that the sea water and it will settle on the bottom of the ocean (Fig. 10.3). Assumption
of stability of sea conditions has been made (Fig. 10.4).