Page 293 - Handbook Of Multiphase Flow Assurance
P. 293
292 10. Research methods in flow assurance
Thermodynamic properties
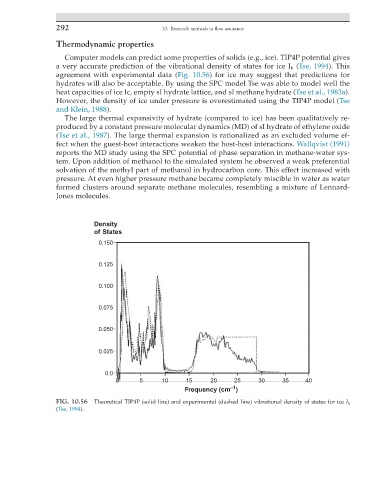
Computer models can predict some properties of solids (e.g., ice). TIP4P potential gives
a very accurate prediction of the vibrational density of states for ice I h (Tse, 1994). This
agreement with experimental data (Fig. 10.56) for ice may suggest that predictions for
hydrates will also be acceptable. By using the SPC model Tse was able to model well the
heat capacities of ice Ic, empty sI hydrate lattice, and sI methane hydrate (Tse et al., 1983a).
However, the density of ice under pressure is overestimated using the TIP4P model (Tse
and Klein, 1988).
The large thermal expansivity of hydrate (compared to ice) has been qualitatively re-
produced by a constant pressure molecular dynamics (MD) of sI hydrate of ethylene oxide
(Tse et al., 1987). The large thermal expansion is rationalized as an excluded volume ef-
fect when the guest-host interactions weaken the host-host interactions. Wallqvist (1991)
reports the MD study using the SPC potential of phase separation in methane-water sys-
tem. Upon addition of methanol to the simulated system he observed a weak preferential
solvation of the methyl part of methanol in hydrocarbon core. This effect increased with
pressure. At even higher pressure methane became completely miscible in water as water
formed clusters around separate methane molecules, resembling a mixture of Lennard-
Jones molecules.
Density
of States
0.150
0.125
0.100
0.075
0.050
0.025
0.0
0 5 10 15 20 25 30 35 40
–1
Frequency (cm )
FIG. 10.56 Theoretical TIP4P (solid line) and experimental (dashed line) vibrational density of states for ice I h
(Tse, 1994).