Page 116 - Handbook of Thermal Analysis of Construction Materials
P. 116
Section 6.0 - Hydration 99
The reactivity of C S also depends on its polymorphic form. A
2
thermal investigation of two C S samples heated to 1400 and 1100°C has
2
indicated that the hydration rate is higher in the sample heated at higher
temperatures. [41]
6.2 Calcium Aluminates
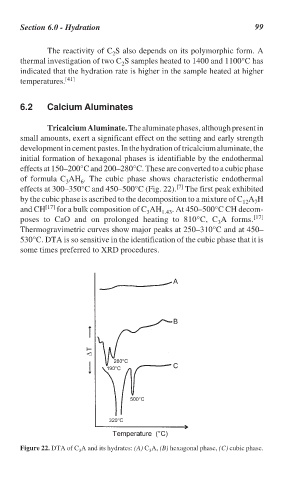
Tricalcium Aluminate. The aluminate phases, although present in
small amounts, exert a significant effect on the setting and early strength
development in cement pastes. In the hydration of tricalcium aluminate, the
initial formation of hexagonal phases is identifiable by the endothermal
effects at 150–200°C and 200–280°C. These are converted to a cubic phase
of formula C AH . The cubic phase shows characteristic endothermal
6
3
[7]
effects at 300–350°C and 450–500°C (Fig. 22). The first peak exhibited
by the cubic phase is ascribed to the decomposition to a mixture of C A H
12
7
and CH [17] for a bulk composition of C AH 1.43 . At 450–500°C CH decom-
3
poses to CaO and on prolonged heating to 810°C, C A forms. [17]
3
Thermogravimetric curves show major peaks at 250–310°C and at 450–
530°C. DTA is so sensitive in the identification of the cubic phase that it is
some times preferred to XRD procedures.
Figure 22. DTA of C A and its hydrates: (A) C A, (B) hexagonal phase, (C) cubic phase.
3 3