Page 137 - Handbook of Thermal Analysis of Construction Materials
P. 137
120 Chapter 3 - Formation and Hydration
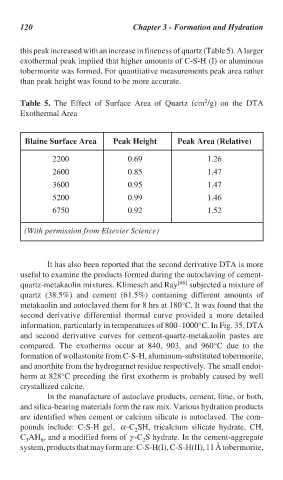
this peak increased with an increase in fineness of quartz (Table 5). A larger
exothermal peak implied that higher amounts of C-S-H (I) or aluminous
tobermorite was formed. For quantitative measurements peak area rather
than peak height was found to be more accurate.
2
Table 5. The Effect of Surface Area of Quartz (cm /g) on the DTA
Exothermal Area
Blaine Surface Area Peak Height Peak Area (Relative)
2200 0.69 1.26
2600 0.85 1.47
3600 0.95 1.47
5200 0.99 1.46
6750 0.92 1.52
(With permission from Elsevier Science)
It has also been reported that the second derivative DTA is more
useful to examine the products formed during the autoclaving of cement-
quartz-metakaolin mixtures. Klimesch and Ray [66] subjected a mixture of
quartz (38.5%) and cement (61.5%) containing different amounts of
metakaolin and autoclaved them for 8 hrs at 180°C. It was found that the
second derivative differential thermal curve provided a more detailed
information, particularly in temperatures of 800–1000°C. In Fig. 35, DTA
and second derivative curves for cement-quartz-metakaolin pastes are
compared. The exotherms occur at 840, 903, and 960°C due to the
formation of wollastonite from C-S-H, aluminum-substituted tobermorite,
and anorthite from the hydrogarnet residue respectively. The small endot-
herm at 828°C preceding the first exotherm is probably caused by well
crystallized calcite.
In the manufacture of autoclave products, cement, lime, or both,
and silica-bearing materials form the raw mix. Various hydration products
are identified when cement or calcium silicate is autoclaved. The com-
pounds include: C-S-H gel, α-C SH, tricalcium silicate hydrate, CH,
2
C AH , and a modified form of γ -C S hydrate. In the cement-aggregate
2
3
6
system, products that may form are: C-S-H(I), C-S-H(II), 11 Å tobermorite,