Page 104 - Handbook of Adhesion Promoters
P. 104
6.4 Modification by epoxy group 97
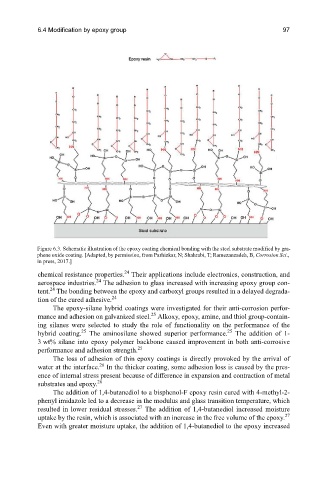
Figure 6.3. Schematic illustration of the epoxy coating chemical bonding with the steel substrate modified by gra-
phene oxide coating. [Adapted, by permission, from Parhizkar, N; Shahrabi, T; Ramezanzadeh, B, Corrosion Sci.,
in press, 2017.]
24
chemical resistance properties. Their applications include electronics, construction, and
24
aerospace industries. The adhesion to glass increased with increasing epoxy group con-
24
tent. The bonding between the epoxy and carboxyl groups resulted in a delayed degrada-
24
tion of the cured adhesive.
The epoxy-silane hybrid coatings were investigated for their anti-corrosion perfor-
25
mance and adhesion on galvanized steel. Alkoxy, epoxy, amine, and thiol group-contain-
ing silanes were selected to study the role of functionality on the performance of the
25
25
hybrid coating. The aminosilane showed superior performance. The addition of 1-
3 wt% silane into epoxy polymer backbone caused improvement in both anti-corrosive
25
performance and adhesion strength.
The loss of adhesion of thin epoxy coatings is directly provoked by the arrival of
26
water at the interface. In the thicker coating, some adhesion loss is caused by the pres-
ence of internal stress present because of difference in expansion and contraction of metal
26
substrates and epoxy.
The addition of 1,4-butanediol to a bisphenol-F epoxy resin cured with 4-methyl-2-
phenyl imidazole led to a decrease in the modulus and glass transition temperature, which
27
resulted in lower residual stresses. The addition of 1,4-butanediol increased moisture
uptake by the resin, which is associated with an increase in the free volume of the epoxy. 27
Even with greater moisture uptake, the addition of 1,4-butanediol to the epoxy increased