Page 259 - Handbook of Adhesives and Sealants
P. 259
228 Chapter Six
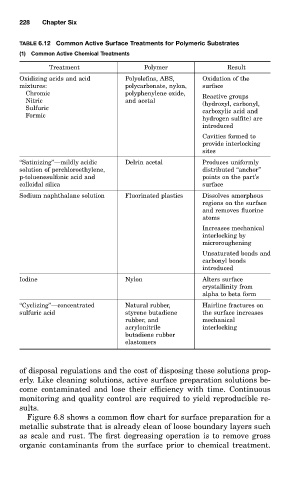
TABLE 6.12 Common Active Surface Treatments for Polymeric Substrates
(1) Common Active Chemical Treatments
Treatment Polymer Result
Oxidizing acids and acid Polyolefins, ABS, Oxidation of the
mixtures: polycarbonate, nylon, surface
Chromic polyphenylene oxide, Reactive groups
Nitric and acetal (hydroxyl, carbonyl,
Sulfuric
carboxylic acid and
Formic
hydrogen sulfite) are
introduced
Cavities formed to
provide interlocking
sites
‘‘Satinizing’’—mildly acidic Delrin acetal Produces uniformly
solution of perchloroethylene, distributed ‘‘anchor’’
p-toluenesulfonic acid and points on the part’s
colloidal silica surface
Sodium naphthalane solution Fluorinated plastics Dissolves amorphous
regions on the surface
and removes fluorine
atoms
Increases mechanical
interlocking by
microroughening
Unsaturated bonds and
carbonyl bonds
introduced
Iodine Nylon Alters surface
crystallinity from
alpha to beta form
‘‘Cyclizing’’—concentrated Natural rubber, Hairline fractures on
sulfuric acid styrene butadiene the surface increases
rubber, and mechanical
acrylonitrile interlocking
butadiene rubber
elastomers
of disposal regulations and the cost of disposing these solutions prop-
erly. Like cleaning solutions, active surface preparation solutions be-
come contaminated and lose their efficiency with time. Continuous
monitoring and quality control are required to yield reproducible re-
sults.
Figure 6.8 shows a common flow chart for surface preparation for a
metallic substrate that is already clean of loose boundary layers such
as scale and rust. The first degreasing operation is to remove gross
organic contaminants from the surface prior to chemical treatment.