Page 76 - Handbook of Adhesives and Sealants
P. 76
44 Chapter One
chemical reaction by crosslinking or curing between the molecular
chains, or by polymerizing from the monomer state. Table 1.13 offers
examples of common adhesives and the changes that are necessary,
after application, for them to solidify and become effective.
Modern synthetic organic based adhesives and sealants will be the
primary topic of this Handbook. However, natural based and mineral
based adhesives and sealants will also be included because they have
wide use in certain applications. Adhesion occurring via metallic pro-
cesses (i.e., welding, soldering, brazing, etc.) will not be included other
than to discuss these as alternative joining methods. Metallic joining
technologies are thoroughly described in other Handbooks.
1.7.2 Manufacturing processes for
adhesives and sealants
Modern adhesives are often a complex formulation of components that
perform specialty functions. The formulation of raw materials into
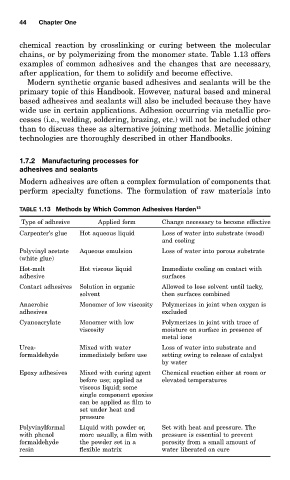
TABLE 1.13 Methods by Which Common Adhesives Harden 13
Type of adhesive Applied form Change necessary to become effective
Carpenter’s glue Hot aqueous liquid Loss of water into substrate (wood)
and cooling
Polyvinyl acetate Aqueous emulsion Loss of water into porous substrate
(white glue)
Hot-melt Hot viscous liquid Immediate cooling on contact with
adhesive surfaces
Contact adhesives Solution in organic Allowed to lose solvent until tacky,
solvent then surfaces combined
Anaerobic Monomer of low viscosity Polymerizes in joint when oxygen is
adhesives excluded
Cyanoacrylate Monomer with low Polymerizes in joint with trace of
viscosity moisture on surface in presence of
metal ions
Urea- Mixed with water Loss of water into substrate and
formaldehyde immediately before use setting owing to release of catalyst
by water
Epoxy adhesives Mixed with curing agent Chemical reaction either at room or
before use; applied as elevated temperatures
viscous liquid; some
single component epoxies
can be applied as film to
set under heat and
pressure
Polyvinylformal Liquid with powder or, Set with heat and pressure. The
with phenol more usually, a film with pressure is essential to prevent
formaldehyde the powder set in a porosity from a small amount of
resin flexible matrix water liberated on cure